RESOURCE ESSENTIALS SUN PROTECTION MINERAL SPF 30- 14% zinc oxide spray
Resource Essentials Sun Protection Mineral SPF 30 by
Drug Labeling and Warnings
Resource Essentials Sun Protection Mineral SPF 30 by is a Otc medication manufactured, distributed, or labeled by Skinresource.md, L.L.C., Skin Resource.MD, L.L.C., Bell International Laboratories. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
INDICATIONS & USAGE
Uses Helps prevent sunburn If used as directed with other sun protection measures, decreases the risk of skin cancer and early skin aging caused by the sun.
Warnings
For external use only
- Flammable Do not spray near heat, sparks, sources of ignition, or flames.
- Protect this product from excessive heat and direct sun
- Do not use on damaged or broken skin
- When using this product keep out of eyes Rinse with water to remove.
- Stop use and ask a doctor if skin irritation or redness occurs for more than 72 hours.
Keep out of reach of children
If product is swallowed, get medical help or contact a Poison Control Center right away
Directions Apply liberally 15 minutes before sun exposure. Reapply: Every 2 hours during the day after swimming or sweating. Children under 6 months: ask a doctor.
Sun Protection Measure: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF value of 15 or higher and other sun protection measures including: Limit time in the sun, especially from 10 a.m.to 2 p.m.
Wear long-sleeved shirts, pants, hats, and sun glasses - ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
 Inactive Ingredients: Aloe Barbadensis Leaf Juice, Bentonite, Bisabolol, Butyloctyl Salicylate, Caprylhydroxamic Acid, Caprylic/Capric Triglyceride, Caprylyl Glycol, Carthamus Tinctorius (Safflower)
Inactive Ingredients: Aloe Barbadensis Leaf Juice, Bentonite, Bisabolol, Butyloctyl Salicylate, Caprylhydroxamic Acid, Caprylic/Capric Triglyceride, Caprylyl Glycol, Carthamus Tinctorius (Safflower)
Seed Oil, Cellulose Gum, Cetearyl Alcohol, Coco-Glucoside, Glycerin, Helianthus Annuus (Sunflower) Oil, Jojoba Esters, Methyl Dihydroabetate, Microcrystalline Cellulose, Polyhydroxystearic Acid, Tocopherol, Water.
-
WARNINGS
 Warnings
Warnings
For external use only - Flammable Do not spray near heat, sparks, sources of ignition, or flames. Protect this product from excessive heat and direct sun
- Do not use on damaged or broken skin
- When using this product keep out of eyes
Rinse with water to remove. - Stop use and ask a doctor if skin irritation or redness occurs for more than 72 hours.
- Keep out of reach of children
lf product is swallowed, get medical help or contact a Poison Control Center right away
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
RESOURCE ESSENTIALS SUN PROTECTION MINERAL SPF 30
14% zinc oxide sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 83694-300 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 24.78 g in 177 g Inactive Ingredients Ingredient Name Strength CARTHAMUS TINCTORIUS (SAFFLOWER) SEED OIL (UNII: 65UEH262IS) HELIANTHUS ANNUUS (SUNFLOWER) SEED OIL (UNII: 3W1JG795YI) BISABOLOL (UNII: 24WE03BX2T) TOCOPHEROL (UNII: R0ZB2556P8) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CELLULOSE GUM (UNII: K679OBS311) CETEARYL ALCOHOL (UNII: 2DMT128M1S) HYDROGENATED JOJOBA OIL, RANDOMIZED (UNII: Q47ST02F58) METHYL DIHYDROABIETATE (UNII: 7666FJ0J9F) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) WATER (UNII: 059QF0KO0R) CAPRYLIC/CAPRIC TRIGLYCERIDE (UNII: C9H2L21V7U) COCO-GLUCOSIDE (UNII: ICS790225B) GLYCERIN (UNII: PDC6A3C0OX) ALOE BARBADENSIS LEAF JUICE (UNII: RUE8E6T4NB) BENTONITE (UNII: A3N5ZCN45C) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 83694-300-01 177 g in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 08/15/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 08/15/2025 Labeler - Skinresource.md, L.L.C. (022050636) Registrant - Skinresource.md, L.L.C. (022050636) Establishment Name Address ID/FEI Business Operations Skin Resource.MD, L.L.C. 022050636 label(83694-300) Establishment Name Address ID/FEI Business Operations Bell International Laboratories 967781555 manufacture(83694-300)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
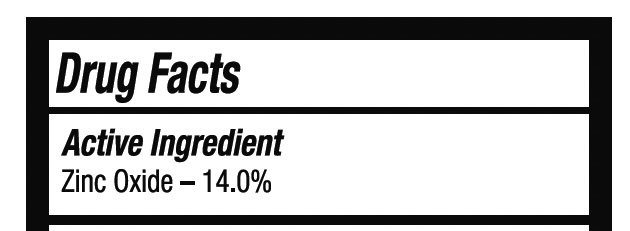 Active Ingredient Zinc Oxide - 14.0%
Active Ingredient Zinc Oxide - 14.0%
 Sunscreen
Sunscreen
 Keep out of reach of children
Keep out of reach of children
 Directions Apply liberally 15 minutes before sun exposure. Reapply: Every 2 hours during the day or after swimming or sweating. Children under 6 months: ask a doctor.
Directions Apply liberally 15 minutes before sun exposure. Reapply: Every 2 hours during the day or after swimming or sweating. Children under 6 months: ask a doctor.
 Resource Essentials
Resource Essentials