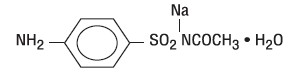
SODIUM SULFACETAMIDE, SULFUR- sodium sulfacetamide 10%, sulfur 5% liquid
SODIUM SULFACETAMIDE, SULFUR by
Drug Labeling and Warnings
SODIUM SULFACETAMIDE, SULFUR by is a Prescription medication manufactured, distributed, or labeled by Aspen Medical LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- CAUTION
-
DOSAGE & ADMINISTRATION
Wash affected areas once or twice daily, or as directed by your physician. Avoid contact with eyes or mucous membranes. Wet skin and leberally apply to areas to be cleansed, massage gently into skin for 10-20 seconds working into a full leather, rinse thoroughly and pat dry. If drying occurs, it may be controlled by rinsing cleanser off sooner or using less often.
FOR EXTERNAL USE ONLY. NOT FOR INTRAVAGINAL OR OPTHALMIC USE. (KEEP AWAY FROM EYES).
KEEP OUT OF REACH OF CHILDREN
(Shake well before use)
- STORAGE AND HANDLING
- DESCRIPTION
-
CLINICAL PHARMACOLOGY
The most widely accepted mechanism of action of sulfonamides is the woods-fields theory which is based on the fact that sulfonamides act as competitive antagonists to para-aminobenzoic acid (FABA), an essential component for bacterial growth. While absorption through intact skin has not been determined, sodium sulfacetamide is readily absorbed from the gastrointestinal tract when taken orally and excreted in the urine, largely unchanged. The biological halflife has variously been reported as 7 to 12.8 hours. The exact mode of action of sulfur in the treatment of acne is unknown but it has been reported that it inhibits the growth of propionibacterium acnes andthe formation of free fatty acids.
- CONTRAINDICATIONS
-
WARNINGS
Although it is rare, sensitivity to sodium sulfacetamide may occur. Therefore, caution and careful supervision should be observed when prescribing this drug for patients who may be prone to hypersensitivity to topical sulfonamides. Systemic toxic reactions such as agranulocytosis, acute hemolytic anemia, purpura hemorrhagica, drug fever, jaundice, and contact dermatitis indicate hypersensitivity to sulfonamides. Particular caution should be employed if areas of denuded or abraded skin are involved.
-
GENERAL
If irritation develops, use of the product should be discontinued and appropriate therapy instituted. Patients should be carefully observed for possible local irritation or sensitization during long-term therapy. The object of this therapy is to achieve desquamation without irritation, but sodium sulfacetamide and sulfur can cause reddening and scaling of the epidermis. These side effects are not unusual in the treatment of acne vulgaris, but patients should be cautioned about the possibility.
INFORMATION FOR PATIENTS
Avoid contact with eyes, eyelids, lips and mucous membranes. If accidental contact occurs, rinse with water. If excessive irritation develops, discontinue use and consult your physician.
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
Long-term studies in animals have not been performed to evaluate carcinogenic potential.
PREGNANCY
Category CAnimal reproduction studies have not been conducted with Sodium Sulfacetamide 10% & Sulfur 5% Cleanser. It is also not known whether Sodium Sulfacetamide 10% & Sulfur 5% Cleanser can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Sodium Sulfacetamide 10% & Sulfur 5% Cleanser should be given to a pregnant woman only if clearly needed.
NURSING MOTHERS
It is not known whether sodium sulfacetamide is excreted in the human milk following topical use of Sodium Sulfacetamide 10% & Sulfur 5% Cleanser. However, small amounts of orally administered sulfonamides have milk. In view of this and because many drugs are excreted in human milk, caution should be exercised when Sodium Sulfacetamide 10% & Sulfur 5% Cleanser is administered to a nursing woman.
- ADVERSE REACTIONS
-
HOW SUPPLIED
Sodium Sulfacetamide 10% & Sulfur 5% Cleanser is available in
6 oz (170 g) bottle NDC: 87026-202-06
12 oz (340 g) bottle NDC: 87026-202-12
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL
Aspen Medical
NDC: 87026-202-06
Rx Only
Sodium
Sulfacetamide
& Sulfur
Sodium Sulfacetamide 10%
Sulfur 5%
10%/5%
Cleanser
For External Use only
NET WT. 6 OZ. (170 g)

-
INGREDIENTS AND APPEARANCE
SODIUM SULFACETAMIDE, SULFUR
sodium sulfacetamide 10%, sulfur 5% liquidProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 87026-202 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFACETAMIDE SODIUM (UNII: 4NRT660KJQ) (SULFACETAMIDE - UNII:4965G3J0F5) SULFACETAMIDE SODIUM 100 mg in 1 g SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 50 mg in 1 g Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SODIUM THIOSULFATE (UNII: HX1032V43M) PHENOXYETHANOL (UNII: HIE492ZZ3T) XANTHAN GUM (UNII: TTV12P4NEE) ALOE VERA LEAF (UNII: ZY81Z83H0X) TRIACETIN (UNII: XHX3C3X673) SODIUM LAURETH SULFATE (UNII: BPV390UAP0) PEG-100 STEARATE (UNII: YD01N1999R) CITRIC ACID (UNII: 2968PHW8QP) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 87026-202-06 170 g in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2026 2 NDC: 87026-202-12 340 g in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2026 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/01/2026 Labeler - Aspen Medical LLC (119562869) Registrant - Aspen Medical LLC (119562869)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.