TRULICITY- dulaglutide injection, solution
Trulicity by
Drug Labeling and Warnings
Trulicity by is a Prescription medication manufactured, distributed, or labeled by Eli Lilly and Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TRULICITY safely and effectively. See full prescribing information for TRULICITY.
TRULICITY (dulaglutide) injection, for subcutaneous use
Initial U.S. Approval: 2014WARNING: RISK OF THYROID C-CELL TUMORS
See full prescribing information for complete boxed warning.
- Dulaglutide causes thyroid C-cell tumors in rats. It is unknown whether TRULICITY causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as the human relevance of dulaglutide-induced rodent thyroid C-cell tumors has not been determined (5.1, 13.1).
- TRULICITY is contraindicated in patients with a personal or family history of MTC and in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk of MTC and symptoms of thyroid tumors (4, 5.1).
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
TRULICITY® is a glucagon-like peptide-1 (GLP-1) receptor agonist indicated:
- as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
- to reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes mellitus who have established cardiovascular disease or multiple cardiovascular risk factors
Limitations of Use:
DOSAGE AND ADMINISTRATION
- Administer once weekly at any time of day (2.1).
- Inject subcutaneously in the abdomen, thigh, or upper arm (2.1).
- Initiate at 0.75 mg subcutaneously once weekly. Dose can be increased to 1.5 mg once weekly for additional glycemic control (2.1).
- If a dose is missed, administer the missed dose as soon as possible if there are at least 3 days (72 hours) until the next scheduled dose (2.1).
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
- TRULICITY is contraindicated in patients with a personal or family history of medullary thyroid carcinoma or in patients with Multiple Endocrine Neoplasia syndrome type 2 (4, 5.1).
- TRULICITY is contraindicated in patients with a prior serious hypersensitivity reaction to TRULICITY or any of the product components (4, 5.4).
WARNINGS AND PRECAUTIONS
- Thyroid C-cell Tumors: See Boxed Warning (5.1).
- Pancreatitis: Has been reported in clinical trials. Discontinue promptly if pancreatitis is suspected. Do not restart if pancreatitis is confirmed. Consider other antidiabetic therapies in patients with history of pancreatitis (5.2).
- Hypoglycemia: When TRULICITY is used with an insulin secretagogue (e.g., a sulfonylurea) or insulin, consider lowering the dose of the sulfonylurea or insulin to reduce the risk of hypoglycemia (5.3).
- Hypersensitivity Reactions: Serious hypersensitivity reactions (e.g., anaphylactic reactions and angioedema) have occurred. Discontinue TRULICITY and promptly seek medical advice (5.4).
- Acute Kidney Injury: Monitor renal function in patients with renal impairment reporting severe adverse gastrointestinal reactions (5.5).
- Severe Gastrointestinal Disease: Use may be associated with gastrointestinal adverse reactions, sometimes severe. Has not been studied in patients with severe gastrointestinal disease and is not recommended in these patients (5.6).
- Diabetic Retinopathy Complications: Have been reported in a cardiovascular outcomes trial. Monitor patients with a history of diabetic retinopathy (5.7).
ADVERSE REACTIONS
The most common adverse reactions, reported in ≥5% of patients treated with TRULICITY are: nausea, diarrhea, vomiting, abdominal pain, and decreased appetite (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Eli Lilly and Company at 1-800-LillyRx (1-800-545-5979) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
- Pregnancy: TRULICITY should be used during pregnancy only if the potential benefit justifies the potential risk to fetus (8.1).
- Renal Impairment: No dosage adjustment recommended. Monitor renal function in patients with renal impairment reporting severe adverse gastrointestinal reactions (5.5, 8.7).
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 2/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: RISK OF THYROID C-CELL TUMORS
1 INDICATIONS AND USAGE
1.1 Limitations of Use
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
2.2 Concomitant Use with an Insulin Secretagogue (e.g., Sulfonylurea) or with Insulin
2.3 Important Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Thyroid C-cell Tumors
5.2 Pancreatitis
5.3 Hypoglycemia with Concomitant Use of Insulin Secretagogues or Insulin
5.4 Hypersensitivity Reactions
5.5 Acute Kidney Injury
5.6 Severe Gastrointestinal Disease
5.7 Diabetic Retinopathy Complications in Patients with a History of Diabetic Retinopathy
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Immunogenicity
6.3 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Oral Medications
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
8.8 Gastroparesis
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Glycemic Control Trials in Adults with Type 2 Diabetes Mellitus
14.2 Cardiovascular Outcomes Trial in Adults with Type 2 Diabetes Mellitus and Cardiovascular Disease or Multiple Cardiovascular Risk Factors
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: RISK OF THYROID C-CELL TUMORS
- In male and female rats, dulaglutide causes a dose-related and treatment-duration-dependent increase in the incidence of thyroid C-cell tumors (adenomas and carcinomas) after lifetime exposure. It is unknown whether TRULICITY causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of dulaglutide-induced rodent thyroid C-cell tumors has not been determined [see Warnings and Precautions (5.1), and Nonclinical Toxicology (13.1)].
- TRULICITY is contraindicated in patients with a personal or family history of MTC and in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk of MTC with use of TRULICITY and inform them of symptoms of thyroid tumors (e.g., mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with TRULICITY [see Contraindications (4) and Warnings and Precautions (5.1)].
-
1 INDICATIONS AND USAGE
TRULICITY® is indicated
- as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
- to reduce the risk of major adverse cardiovascular events (cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke) in adults with type 2 diabetes mellitus who have established cardiovascular disease or multiple cardiovascular risk factors.
1.1 Limitations of Use
- TRULICITY has not been studied in patients with a history of pancreatitis [see Warnings and Precautions (5.2)]. Consider other antidiabetic therapies in patients with a history of pancreatitis.
- TRULICITY should not be used in patients with type 1 diabetes mellitus or for the treatment of diabetic ketoacidosis. TRULICITY is not a substitute for insulin.
- TRULICITY has not been studied in patients with severe gastrointestinal disease, including severe gastroparesis. The use of TRULICITY is not recommended in patients with pre-existing severe gastrointestinal disease [see Warnings and Precautions (5.6)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
The recommended initiating dose of TRULICITY is 0.75 mg once weekly. The dose may be increased to 1.5 mg once weekly for additional glycemic control. The maximum recommended dose is 1.5 mg once weekly.
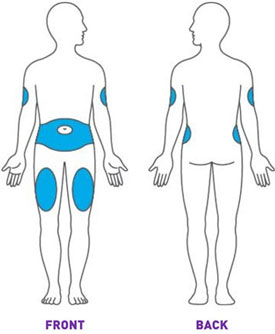
Administer TRULICITY once weekly, any time of day, with or without food. TRULICITY should be injected subcutaneously in the abdomen, thigh, or upper arm.
If a dose is missed, instruct patients to administer as soon as possible if there are at least 3 days (72 hours) until the next scheduled dose. If less than 3 days remain before the next scheduled dose, skip the missed dose and administer the next dose on the regularly scheduled day. In each case, patients can then resume their regular once weekly dosing schedule.
The day of weekly administration can be changed if necessary as long as the last dose was administered 3 or more days before.
2.2 Concomitant Use with an Insulin Secretagogue (e.g., Sulfonylurea) or with Insulin
When initiating TRULICITY, consider reducing the dosage of concomitantly administered insulin secretagogues (e.g., sulfonylureas) or insulin to reduce the risk of hypoglycemia [see Warnings and Precautions (5.3)].
2.3 Important Administration Instructions
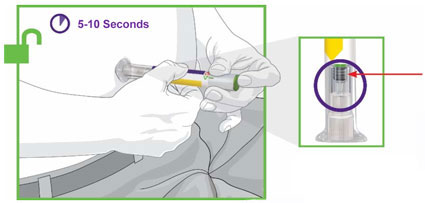
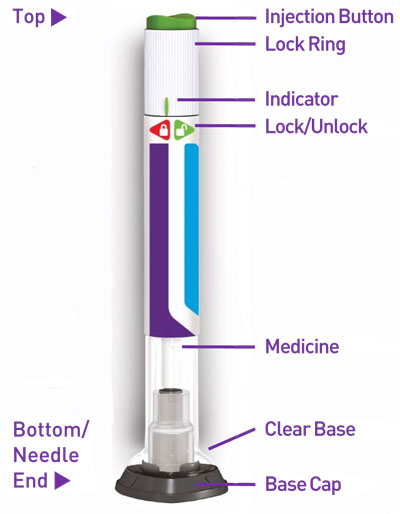
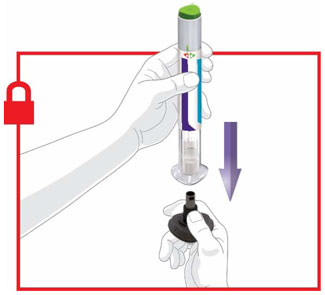
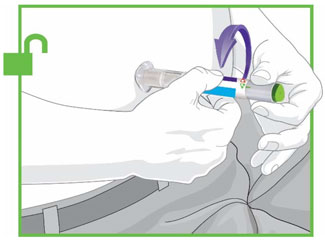
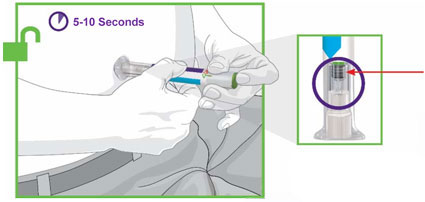
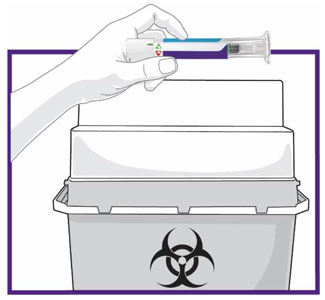
Prior to initiation of TRULICITY, patients should be trained by their healthcare professional on proper injection technique. Training reduces the risk of administration errors such as improper injection site, needle sticks, and incomplete dosing. Refer to the accompanying Instructions for Use for complete administration instructions with illustrations. The instructions can also be found at www.trulicity.com.
When using TRULICITY with insulin, instruct patients to administer as separate injections and to never mix the products. It is acceptable to inject TRULICITY and insulin in the same body region but the injections should not be adjacent to each other.
When injecting in the same body region, advise patients to use a different injection site each week. TRULICITY must not be administered intravenously or intramuscularly.
TRULICITY solution should be visually inspected for particulate matter and discoloration prior to administration.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Medullary Thyroid Carcinoma
TRULICITY is contraindicated in patients with a personal or family history of medullary thyroid carcinoma (MTC) or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2) [see Warnings and Precautions (5.1)].
Hypersensitivity
TRULICITY is contraindicated in patients with a prior serious hypersensitivity reaction to dulaglutide or to any of the product components. Serious hypersensitivity reactions including anaphylactic reactions and angioedema have been reported with TRULICITY [see Warnings and Precautions (5.4)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Thyroid C-cell Tumors
In male and female rats, dulaglutide causes a dose-related and treatment-duration-dependent increase in the incidence of thyroid C-cell tumors (adenomas and carcinomas) after lifetime exposure [see Nonclinical Toxicology (13.1)]. Glucagon-like peptide-1 (GLP-1) receptor agonists have induced thyroid C-cell adenomas and carcinomas in mice and rats at clinically relevant exposures. It is unknown whether TRULICITY will cause thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans, as the human relevance of dulaglutide-induced rodent thyroid C-cell tumors has not been determined.
One case of MTC was reported in a patient treated with TRULICITY in the phase 3 clinical program. This patient had pretreatment calcitonin levels approximately 8 times the upper limit of normal (ULN). An additional case of C-cell hyperplasia with elevated calcitonin levels following treatment was reported in the cardiovascular outcomes trial (REWIND). Cases of MTC in patients treated with liraglutide, another GLP-1 receptor agonist, have been reported in the postmarketing period; the data in these reports are insufficient to establish or exclude a causal relationship between MTC and GLP-1 receptor agonist use in humans.
TRULICITY is contraindicated in patients with a personal or family history of MTC or in patients with MEN 2. Counsel patients regarding the potential risk for MTC with the use of TRULICITY and inform them of symptoms of thyroid tumors (e.g. a mass in the neck, dysphagia, dyspnea, persistent hoarseness).
Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with TRULICITY. Such monitoring may increase the risk of unnecessary procedures, due to the low test specificity for serum calcitonin and a high background incidence of thyroid disease. Significantly elevated serum calcitonin values may indicate MTC and patients with MTC usually have calcitonin values >50 ng/L. If serum calcitonin is measured and found to be elevated, the patient should be further evaluated. Patients with thyroid nodules noted on physical examination or neck imaging should also be further evaluated.
5.2 Pancreatitis
In Phase 2 and Phase 3 clinical studies, 12 (3.4 cases per 1000 patient years) pancreatitis-related adverse reactions were reported in patients exposed to TRULICITY versus 3 in non-incretin comparators (2.7 cases per 1000 patient years). An analysis of adjudicated events revealed 5 cases of confirmed pancreatitis in patients exposed to TRULICITY (1.4 cases per 1000 patient years) versus 1 case in non-incretin comparators (0.88 cases per 1000 patient years).
After initiation of TRULICITY, observe patients carefully for signs and symptoms of pancreatitis, including persistent severe abdominal pain, sometimes radiating to the back, which may or may not be accompanied by vomiting. If pancreatitis is suspected, promptly discontinue TRULICITY. If pancreatitis is confirmed, TRULICITY should not be restarted. TRULICITY has not been evaluated in patients with a prior history of pancreatitis. Consider other antidiabetic therapies in patients with a history of pancreatitis.
5.3 Hypoglycemia with Concomitant Use of Insulin Secretagogues or Insulin
The risk of hypoglycemia is increased when TRULICITY is used in combination with insulin secretagogues (e.g., sulfonylureas) or insulin. Patients may require a lower dose of sulfonylurea or insulin to reduce the risk of hypoglycemia in this setting [see Dosage and Administration (2.2), Adverse Reactions (6.1)].
5.4 Hypersensitivity Reactions
There have been postmarketing reports of serious hypersensitivity reactions including anaphylactic reactions and angioedema in patients treated with TRULICITY [see Adverse Reactions (6.3)]. If a hypersensitivity reaction occurs, discontinue TRULICITY; treat promptly per standard of care, and monitor until signs and symptoms resolve. Do not use in patients with a previous hypersensitivity reaction to TRULICITY [see Contraindications (4)].
Anaphylaxis and angioedema have been reported with other GLP-1 receptor agonists. Use caution in a patient with a history of angioedema or anaphylaxis with another GLP-1 receptor agonist because it is unknown whether such patients will be predisposed to anaphylaxis with TRULICITY.
5.5 Acute Kidney Injury
In patients treated with GLP-1 receptor agonists, including TRULICITY, there have been postmarketing reports of acute renal failure and worsening of chronic renal failure, which may sometimes require hemodialysis. Some of these events were reported in patients without known underlying renal disease. A majority of reported events occurred in patients who had experienced nausea, vomiting, diarrhea, or dehydration. Because these reactions may worsen renal function, use caution when initiating or escalating doses of TRULICITY in patients with renal impairment. Monitor renal function in patients with renal impairment reporting severe adverse gastrointestinal reactions [see Use in Specific Populations (8.7)].
5.6 Severe Gastrointestinal Disease
Use of TRULICITY may be associated with gastrointestinal adverse reactions, sometimes severe [see Adverse Reactions (6.1)]. TRULICITY has not been studied in patients with severe gastrointestinal disease, including severe gastroparesis, and is therefore not recommended in these patients.
5.7 Diabetic Retinopathy Complications in Patients with a History of Diabetic Retinopathy
In a cardiovascular outcomes trial with a median follow up of 5.4 years involving patients with type 2 diabetes with established cardiovascular disease or multiple cardiovascular risk factors, diabetic retinopathy complications occurred in patients treated with TRULICITY 1.5 mg (1.9%) and placebo (1.5%). These events were prospectively ascertained as a secondary composite endpoint. The proportion of patients with diabetic retinopathy complications was larger among patients with a history of diabetic retinopathy at baseline (TRULICITY 8.5%, placebo 6.2%) than among patients without a known history of diabetic retinopathy (TRULICITY 1.0%, placebo 1.0%).
Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy. Patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy.
-
6 ADVERSE REACTIONS
The following serious reactions are described below or elsewhere in the prescribing information:
- Risk of Thyroid C-cell Tumors [see Warnings and Precautions (5.1)]
- Pancreatitis [see Warnings and Precautions (5.2)]
- Hypoglycemia with Concomitant Use of Insulin Secretagogues or Insulin [see Warnings and Precautions (5.3)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.4)]
- Acute Kidney Injury [see Warnings and Precautions (5.5)]
- Severe Gastrointestinal Disease [see Warnings and Precautions (5.6)]
- Diabetic Retinopathy Complications in Patients with a History of Diabetic Retinopathy [see Warnings and Precautions (5.7)]
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Pool of Placebo-controlled Trials
The data in Table 1 are derived from placebo-controlled trials [see Clinical Studies (14)].
These data reflect exposure of 1670 patients to TRULICITY and a mean duration of exposure to TRULICITY of 23.8 weeks. Across the treatment arms, the mean age of patients was 56 years, 1% were 75 years or older and 53% were male. The population in these studies was 69% White, 7% Black or African American, 13% Asian; 30% were of Hispanic or Latino ethnicity. At baseline, the population had diabetes for an average of 8.0 years and had a mean HbA1c of 8.0%. At baseline, 2.5% of the population reported retinopathy. Baseline estimated renal function was normal or mildly impaired (eGFR ≥60 mL/min/1.73 m2) in 96.0% of the pooled study populations.
Table 1 shows common adverse reactions, excluding hypoglycemia, associated with the use of TRULICITY in a pool of placebo-controlled trials. These adverse reactions were not present at baseline, occurred more commonly on TRULICITY than on placebo, and occurred in at least 5% of patients treated with TRULICITY.
Table 1: Adverse Reactions in Placebo-Controlled Trials Reported in ≥5% of TRULICITY-Treated Patients a Includes diarrhea, fecal volume increased, frequent bowel movements.
b Includes retching, vomiting, vomiting projectile.
c Includes abdominal discomfort, abdominal pain, abdominal pain lower, abdominal pain upper, abdominal tenderness, gastrointestinal pain.
d Includes fatigue, asthenia, malaise.
Note: Percentages reflect the number of patients that reported at least 1 treatment-emergent occurrence of the adverse reaction.
Adverse Reaction Placebo
(N=568)
%TRULICITY 0.75 mg
(N=836)
%TRULICITY 1.5 mg
(N=834)
%Nausea 5.3 12.4 21.1 Diarrheaa 6.7 8.9 12.6 Vomitingb 2.3 6.0 12.7 Abdominal Painc 4.9 6.5 9.4 Decreased Appetite 1.6 4.9 8.6 Dyspepsia 2.3 4.1 5.8 Fatigued 2.6 4.2 5.6 Gastrointestinal Adverse Reactions
In the pool of placebo-controlled trials, gastrointestinal adverse reactions occurred more frequently among patients receiving TRULICITY than placebo (placebo 21.3%, 0.75 mg 31.6%, 1.5 mg 41.0%). More patients receiving TRULICITY 0.75 mg (1.3%) and TRULICITY 1.5 mg (3.5%) discontinued treatment due to gastrointestinal adverse reactions than patients receiving placebo (0.2%). Investigators graded the severity of gastrointestinal adverse reactions occurring on 0.75 mg and 1.5 mg of TRULICITY as “mild” in 58% and 48% of cases, respectively, “moderate” in 35% and 42% of cases, respectively, or “severe” in 7% and 11% of cases, respectively.
In addition to the reactions in Table 1, the following adverse reactions were reported more frequently in TRULICITY-treated patients than placebo (frequencies listed, respectively, as: placebo; 0.75 mg; 1.5 mg): constipation (0.7%, 3.9%, 3.7%), flatulence (1.4%, 1.4%, 3.4%), abdominal distension (0.7%, 2.9%, 2.3%), gastroesophageal reflux disease (0.5%, 1.7%, 2.0%), and eructation (0.2%, 0.6%, 1.6%).
Pool of Placebo- and Active-Controlled Trials
The occurrence of adverse reactions was also evaluated in a larger pool of patients with type 2 diabetes participating in 6 placebo- and active-controlled trials evaluating the use of TRULICITY as monotherapy and add-on therapy to oral medications or insulin [see Clinical Studies (14)]. In this pool, a total of 3342 patients with type 2 diabetes were treated with TRULICITY for a mean duration of 52 weeks. The mean age of patients was 56 years, 2% were 75 years or older and 51% were male. The population in these studies was 71% White, 7% Black or African American, 11% Asian; 32% were of Hispanic or Latino ethnicity. At baseline, the population had diabetes for an average of 8.2 years and had a mean HbA1c of 7.6-8.5%. At baseline, 5.2% of the population reported retinopathy. Baseline estimated renal function was normal or mildly impaired (eGFR ≥60 mL/min/1.73 m2) in 95.7% of the TRULICITY population.
In the pool of placebo- and active-controlled trials, the types and frequency of common adverse reactions, excluding hypoglycemia, were similar to those listed in Table 1.
Other Adverse Reactions
Hypoglycemia
Table 2 summarizes the incidence of hypoglycemia in the placebo-controlled clinical studies: episodes with a glucose level <54 mg/dL with or without symptoms, and severe hypoglycemia, defined as an episode requiring the assistance of another person to actively administer carbohydrate, glucagon, or other resuscitative actions.
Table 2: Incidence (%) of Hypoglycemia in Placebo-Controlled Trials Placebo TRULICITY 0.75 mg TRULICITY 1.5 mg Add-on to Metformin (26 weeks) N=177 N=302 N=304 Hypoglycemia with a glucose level <54 mg/dL 0 0.3 0.7 Severe hypoglycemia 0 0 0 Add-on to Metformin + Pioglitazone (26 weeks) N=141 N=280 N=279 Hypoglycemia with a glucose level <54 mg/dL 1.4 2.1 0 Severe hypoglycemia 0 0 0 Add-on to Glimepiride (24 weeks) N=60 - N=239 Hypoglycemia with a glucose level <54 mg/dL 0 - 3.3 Severe hypoglycemia 0 - 0 In Combination with Insulin Glargine ± Metformin (28 weeks) N=150 - N=150 Hypoglycemia with a glucose level <54 mg/dL 9.3 - 14.7 Severe hypoglycemia 0 - 0.7 Add-on to SGLT2i ± Metformin (24 weeks) N=140 N=141 N=142 Hypoglycemia with a glucose level <54 mg/dL 0.7 0.7 0.7 Severe hypoglycemia 0 0.7 0 Hypoglycemia was more frequent when TRULICITY was used in combination with a sulfonylurea or insulin than when used with non-secretagogues [see Warnings and Precautions (5.3)]. In a 78-week clinical trial, hypoglycemia (glucose level <54 mg/dL) occurred in 20% and 21% of patients when TRULICITY 0.75 mg and 1.5 mg, respectively, were co-administered with a sulfonylurea. Severe hypoglycemia occurred in 0% and 0.7% of patients when TRULICITY 0.75 mg and 1.5 mg, respectively, were co-administered with a sulfonylurea. In a 52-week clinical trial, hypoglycemia (glucose level <54 mg/dL) occurred in 77% and 69% of patients when TRULICITY 0.75 mg and 1.5 mg, respectively, were co-administered with prandial insulin. Severe hypoglycemia occurred in 2.7% and 3.4% of patients when TRULICITY 0.75 mg and 1.5 mg, respectively, were co-administered with prandial insulin. Refer to Table 2 for the incidence of hypoglycemia in patients treated in combination with basal insulin glargine.
Cholelithiasis and Cholecystitis
In a cardiovascular outcomes trial with a median follow up of 5.4 years, cholelithiasis occurred at a rate of 0.62/100 patient-years in TRULICITY-treated patients and 0.56/100 patient-years in placebo-treated patients after adjusting for prior cholecystectomy. Serious events of acute cholecystitis were reported in 0.5% and 0.3% of patients on TRULICITY and placebo respectively.
Heart Rate Increase and Tachycardia-Related Adverse Reactions
TRULICITY 0.75 mg and 1.5 mg resulted in a mean increase in heart rate (HR) of 2-4 beats per minute (bpm).
Adverse reactions of sinus tachycardia were reported more frequently in patients exposed to TRULICITY. Sinus tachycardia was reported in 3.0%, 2.8%, and 5.6% of patients treated with placebo, TRULICITY 0.75 mg and TRULICITY 1.5 mg, respectively. Persistence of sinus tachycardia (reported at more than 2 visits) was reported in 0.2%, 0.4% and 1.6% of patients treated with placebo, TRULICITY 0.75 mg and TRULICITY 1.5 mg, respectively. Episodes of sinus tachycardia, associated with a concomitant increase from baseline in heart rate of ≥15 beats per minute, were reported in 0.7%, 1.3% and 2.2% of patients treated with placebo, TRULICITY 0.75 mg and TRULICITY 1.5 mg, respectively.
Hypersensitivity
Systemic hypersensitivity adverse reactions, sometimes severe (e.g., severe urticaria, systemic rash, facial edema, lip swelling), occurred in 0.5% of patients on TRULICITY in the four Phase 2 and five Phase 3 studies.
Injection-site Reactions
In the placebo-controlled studies, injection-site reactions (e.g., injection-site rash, erythema) were reported in 0.5% of TRULICITY-treated patients and in 0.0% of placebo-treated patients.
PR Interval Prolongation and Adverse Reactions of First-Degree Atrioventricular (AV) Block
A mean increase from baseline in PR interval of 2-3 milliseconds was observed in TRULICITY-treated patients in contrast to a mean decrease of 0.9 milliseconds in placebo-treated patients. The adverse reaction of first-degree AV block occurred more frequently in patients treated with TRULICITY than placebo (0.9%, 1.7% and 2.3% for placebo, TRULICITY 0.75 mg and TRULICITY 1.5 mg, respectively). On electrocardiograms, a PR interval increase to at least 220 milliseconds was observed in 0.7%, 2.5% and 3.2% of patients treated with placebo, TRULICITY 0.75 mg and TRULICITY 1.5 mg, respectively.
6.2 Immunogenicity
Across four Phase 2 and five Phase 3 clinical studies, 64 (1.6%) TRULICITY-treated patients developed anti-drug antibodies (ADAs) to the active ingredient in TRULICITY (i.e., dulaglutide).
Of the 64 dulaglutide-treated patients that developed dulaglutide ADAs, 34 patients (0.9% of the overall population) had dulaglutide-neutralizing antibodies, and 36 patients (0.9% of the overall population) developed antibodies against native GLP-1.
The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, the incidence of antibodies to dulaglutide cannot be directly compared with the incidence of antibodies of other products.
6.3 Postmarketing Experience
The following additional adverse reactions have been reported during post-approval use of TRULICITY. Because these events are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Anaphylactic reactions, angioedema [see Contraindications (4), Warnings and Precautions (5.4), Patient Counseling Information (17)].
- Acute renal failure or worsening of chronic renal failure, sometimes requiring hemodialysis [see Warnings and Precautions (5.5) and Patient Counseling Information (17)].
-
7 DRUG INTERACTIONS
7.1 Oral Medications
TRULICITY slows gastric emptying and thus has the potential to reduce the rate of absorption of concomitantly administered oral medications. Caution should be exercised when oral medications are concomitantly administered with TRULICITY. Drug levels of oral medications with a narrow therapeutic index should be adequately monitored when concomitantly administered with TRULICITY. In clinical pharmacology studies, TRULICITY did not affect the absorption of the tested, orally administered medications to a clinically relevant degree [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited data with TRULICITY in pregnant women are not sufficient to determine a drug-associated risk for major birth defects and miscarriage. There are clinical considerations regarding the risks of poorly controlled diabetes in pregnancy [see Clinical Considerations]. Based on animal reproduction studies, there may be risks to the fetus from exposure to dulaglutide during pregnancy. TRULICITY should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
In pregnant rats administered dulaglutide during organogenesis, early embryonic deaths, fetal growth reductions, and fetal abnormalities occurred at systemic exposures at least 14-times human exposure at the maximum recommended human dose (MRHD) of 1.5 mg/week. In pregnant rabbits administered dulaglutide during organogenesis, major fetal abnormalities occurred at 13-times human exposure at the MRHD. Adverse embryo/fetal effects in animals occurred in association with decreased maternal weight and food consumption attributed to the pharmacology of dulaglutide [see Data].
The estimated background risk of major birth defects is 6–10% in women with pre-gestational diabetes with an HbA1c >7% and has been reported to be as high as 20–25% in women with an HbA1c >10%. The estimated background risk of miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Poorly controlled diabetes in pregnancy increases the maternal risk for diabetic ketoacidosis, pre-eclampsia, spontaneous abortions, preterm delivery, stillbirth and delivery complications. Poorly controlled diabetes increases the fetal risk for major birth defects, stillbirth, and macrosomia-related morbidity.
Data
Animal Data
Pregnant rats given subcutaneous doses of 0.49, 1.63, or 4.89 mg/kg dulaglutide every 3 days during organogenesis had systemic exposures 4-, 14-, and 44-times human exposure at the maximum recommended human dose (MRHD) of 1.5 mg/week, respectively, based on plasma area under the time-concentration curve (AUC) comparison. Reduced fetal weights associated with decreased maternal food intake and decreased weight gain attributed to the pharmacology of dulaglutide were observed at ≥1.63 mg/kg. Irregular skeletal ossifications and increases in post-implantation loss also were observed at 4.89 mg/kg.
In pregnant rabbits given subcutaneous doses of 0.04, 0.12, or 0.41 mg/kg dulaglutide every 3 days during organogenesis, systemic exposures in pregnant rabbits were 1-, 4-, and 13-times human exposure at the MRHD, based on plasma AUC comparison. Fetal visceral malformation of lung lobular agenesis and skeletal malformations of the vertebrae and/or ribs were observed in conjunction with decreased maternal food intake and decreased weight gain attributed to the pharmacology of dulaglutide at 0.41 mg/kg.
In a prenatal-postnatal study in F0 maternal rats given subcutaneous doses of 0.2, 0.49, or 1.63 mg/kg every third day from implantation through lactation, systemic exposures in pregnant rats were 2-, 4-, and 16-times human exposure at the MRHD, based on plasma AUC comparison. F1 pups from F0 maternal rats given 1.63 mg/kg dulaglutide had statistically significantly lower mean body weight from birth through postnatal day 63 for males and postnatal day 84 for females. F1 offspring from F0 maternal rats receiving 1.63 mg/kg dulaglutide had decreased forelimb and hindlimb grip strength and males had delayed balano-preputial separation. Females had decreased startle response. These physical findings may relate to the decreased size of the offspring relative to controls as they appeared at early postnatal assessments but were not observed at a later assessment. F1 female offspring of the F0 maternal rats given 1.63 mg/kg of dulaglutide had a longer mean escape time and a higher mean number of errors relative to concurrent control during 1 of 2 trials in the memory evaluation portion of the Biel water maze. These findings occurred in conjunction with decreased F0 maternal food intake and decreased weight gain attributed to the pharmacologic activity at 1.63 mg/kg. The human relevance of these memory deficits in the F1 female rats is not known.
8.2 Lactation
Risk Summary
There are no data on the presence of dulaglutide in human milk, the effects on the breastfed infant, or the effects on milk production. The presence of dulaglutide in milk of treated lactating animals was not determined. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for TRULICITY and any potential adverse effects on the breastfed infant from TRULICITY or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness of TRULICITY have not been established in pediatric patients. TRULICITY is not recommended for use in pediatric patients younger than 18 years.
8.5 Geriatric Use
In the glycemic control trials [see Adverse Reactions (6.1)], 620 (18.6%) TRULICITY-treated patients were 65 years of age or older and 65 (1.9%) TRULICITY-treated patients were 75 years of age or older at baseline. No overall differences in safety or efficacy were detected between these patients and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
In the TRULICITY 1.5 mg treatment arm of the REWIND trial [see Clinical Studies (14.2)], a total of 2619 (52.9%) patients were 65 years of age or older, and 484 (9.8%) patients were 75 years of age or older at baseline. No overall differences in safety or efficacy were observed based on age.
8.6 Hepatic Impairment
There is limited clinical experience in patients with mild, moderate, or severe hepatic impairment. Therefore, TRULICITY should be used with caution in these patient populations.
In a clinical pharmacology study in subjects with varying degrees of hepatic impairment, no clinically relevant change in dulaglutide pharmacokinetics (PK) was observed [see Clinical Pharmacology (12.3)].
8.7 Renal Impairment
In four Phase 2 and five Phase 3 randomized clinical studies, at baseline, 50 (1.2%) TRULICITY-treated patients had mild renal impairment (eGFR ≥60 but <90 mL/min/1.73 m2), 171 (4.3%) TRULICITY-treated patients had moderate renal impairment (eGFR ≥30 but <60 mL/min/1.73 m2), and no TRULICITY-treated patients had severe renal impairment (eGFR <30 mL/min/1.73 m2). In a 52-week clinical trial, 270 (71%) TRULICITY-treated patients had moderate renal impairment (eGFR ≥ 30 but <60 mL/min/1.73 m2) and 112 (29%) TRULICITY-treated patients had severe renal impairment (eGFR ≥ 15 but <30 mL/min/1.73 m2) [see Clinical Studies (14.1)]. No overall differences in safety or effectiveness were observed in this study.
In the TRULICITY 1.5 mg arm of the REWIND trial [see Clinical Studies (14.2)], 2435 (50.2%) patients had mild renal impairment, 1031 (21.2%) patients had moderate renal impairment, and 50 (1.0%) patients had severe renal impairment at baseline. No overall differences in safety or efficacy were observed between patients with moderate to severe renal impairment (eGFR <60 mL/min/1.73 m2) and patients with mild or no renal impairment (eGFR ≥60 mL/min/1.73 m2).
In a clinical pharmacology study in subjects with renal impairment, including end-stage renal disease (ESRD), no clinically relevant change in dulaglutide PK was observed. In the 52-week Phase 3 study in patients with type 2 diabetes and moderate to severe renal impairment, the PK behavior of TRULICITY 0.75 mg and 1.5 mg once weekly was similar to that demonstrated in previous clinical studies [see Clinical Pharmacology (12.3)].
No dose adjustment is recommended in patients with renal impairment including end-stage renal disease (ESRD). Monitor renal function in patients with renal impairment reporting severe adverse gastrointestinal reactions. There is limited clinical experience in patients with ESRD. TRULICITY should be used with caution in patients with ESRD [see Warning and Precautions (5.5), Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Overdoses have been reported in clinical studies. Effects associated with these overdoses were primarily mild or moderate gastrointestinal events (e.g., nausea, vomiting) and non-severe hypoglycemia. In the event of overdose, appropriate supportive care (including frequent plasma glucose monitoring) should be initiated according to the patient's clinical signs and symptoms.
-
11 DESCRIPTION
TRULICITY contains dulaglutide, a human GLP-1 receptor agonist. The molecule is a fusion protein that consists of 2 identical, disulfide-linked chains, each containing an N-terminal GLP-1 analog sequence covalently linked to the Fc portion of a modified human immunoglobulin G4 (IgG4) heavy chain by a small peptide linker and is produced using mammalian cell culture. The GLP-1 analog portion of dulaglutide is 90% homologous to native human GLP-1 (7-37). Structural modifications were introduced in the GLP-1 part of the molecule responsible for interaction with the enzyme dipeptidyl-peptidase-IV (DPP-4). Additional modifications were made in an area with a potential T-cell epitope and in the areas of the IgG4 Fc part of the molecule responsible for binding the high-affinity Fc receptors and half-antibody formation. The overall molecular weight of dulaglutide is approximately 63 kilodaltons.
TRULICITY is a clear, colorless, sterile solution. Each 0.5 mL of TRULICITY solution contains 0.75 mg or 1.5 mg of dulaglutide. Each single-dose pen contains 0.5 mL of solution and the following excipients: citric acid anhydrous (0.07 mg), mannitol (23.2 mg), polysorbate 80 (0.10 mg), trisodium citrate dihydrate (1.37 mg), in water for injection.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
TRULICITY contains dulaglutide, which is a human GLP-1 receptor agonist with 90% amino acid sequence homology to endogenous human GLP-1 (7-37). Dulaglutide activates the GLP-1 receptor, a membrane-bound cell-surface receptor coupled to adenylyl cyclase in pancreatic beta cells. Dulaglutide increases intracellular cyclic AMP (cAMP) in beta cells leading to glucose-dependent insulin release. Dulaglutide also decreases glucagon secretion and slows gastric emptying.
12.2 Pharmacodynamics
TRULICITY lowers fasting glucose and reduces postprandial glucose (PPG) concentrations in patients with type 2 diabetes mellitus. The reduction in fasting and postprandial glucose can be observed after a single dose.
Fasting and Postprandial Glucose
In a clinical pharmacology study in adults with type 2 diabetes mellitus, treatment with once weekly TRULICITY resulted in a reduction of fasting and 2-hour PPG concentrations, and postprandial serum glucose incremental AUC, when compared to placebo (-25.6 mg/dL, -59.5 mg/dL, and -197 mg*h/dL, respectively); these effects were sustained after 6 weeks of dosing with the 1.5 mg dose.
First- and Second-Phase Insulin Secretion
Both first- and second-phase insulin secretion were increased in patients with type 2 diabetes treated with TRULICITY compared with placebo.
Insulin and Glucagon Secretion
TRULICITY stimulates glucose-dependent insulin secretion and reduces glucagon secretion. Treatment with TRULICITY 0.75 mg and 1.5 mg once weekly increased fasting insulin from baseline at Week 26 by 35.38 and 17.50 pmol/L, respectively, and C-peptide concentration by 0.09 and 0.07 nmol/L, respectively, in a Phase 3 monotherapy study. In the same study, fasting glucagon concentration was reduced by 1.71 and 2.05 pmol/L from baseline with TRULICITY 0.75 mg and 1.5 mg, respectively.
12.3 Pharmacokinetics
The pharmacokinetics of dulaglutide is similar between healthy subjects and patients with type 2 diabetes mellitus. Following subcutaneous administration, the time to maximum plasma concentration of dulaglutide at steady state ranges from 24 to 72 hours, with a median of 48 hours. After multiple-dose administration of 1.5 mg to steady state, the mean peak plasma concentration (Cmax) and total systemic exposure (AUC) of dulaglutide were 114 ng/mL (range 56 to 231 ng/mL) and 14,000 ng*h/mL (range 6940 to 26,000 ng*h/mL), respectively; accumulation ratio was approximately 1.56. Steady-state plasma dulaglutide concentrations were achieved between 2 and 4 weeks following once weekly administration. Site of subcutaneous administration (abdomen, upper arm, and thigh) had no statistically significant effect on the exposure to dulaglutide.
Absorption – The mean absolute bioavailability of dulaglutide following subcutaneous administration of single 0.75 mg and 1.5 mg doses was 65% and 47%, respectively.
Distribution – The mean volumes of distribution after subcutaneous administration of TRULICITY 0.75 mg and 1.5 mg to steady state were approximately 19.2 L (range 14.3 to 26.4 L) and 17.4 L (range 9.3 to 33 L), respectively.
Metabolism – Dulaglutide is presumed to be degraded into its component amino acids by general protein catabolism pathways.
Elimination – The mean apparent clearance at steady state of dulaglutide is approximately 0.111 L/h for the 0.75 mg dose, and 0.107 L/h for the 1.5 mg dose. The elimination half-life of dulaglutide for both doses is approximately 5 days.
Specific Populations
No dose adjustment of dulaglutide is needed based on age, gender, race, ethnicity, body weight, or renal or hepatic impairment. The effects of intrinsic factors on the PK of dulaglutide are shown in Figure 1.
Renal – Dulaglutide systemic exposure was increased by 20, 28, 14 and 12% for mild, moderate, severe, and ESRD renal impairment sub-groups, respectively, compared to subjects with normal renal function. The corresponding values for increase in Cmax were 13, 23, 20 and 11%, respectively (Figure 1). Additionally, in a 52 week Phase 3 study in patients with type 2 diabetes and moderate to severe renal impairment, the PK behavior of TRULICITY 0.75 mg and 1.5 mg once weekly was similar to that demonstrated in previous clinical studies [see Warning and Precautions (5.5), Use in Specific Population (8.7)].
Hepatic – Dulaglutide systemic exposure decreased by 23, 33 and 21% for mild, moderate and severe hepatic impairment groups, respectively, compared to subjects with normal hepatic function, and Cmax was decreased by a similar magnitude (Figure 1). [see Use in Specific Population (8.6)].
Drug Interactions
The potential effect of co-administered medications on the PK of dulaglutide and vice versa was studied in several single- and multiple-dose studies in healthy subjects, patients with type 2 diabetes mellitus, and patients with hypertension.
Potential for Dulaglutide to Influence the Pharmacokinetics of Other Drugs
Dulaglutide slows gastric emptying and, as a result, may reduce the extent and rate of absorption of orally co-administered medications. In clinical pharmacology studies, dulaglutide did not affect the absorption of the tested orally administered medications to any clinically relevant degree.
Pharmacokinetic (PK) measures indicating the magnitude of these interactions are presented in Figure 2. No dose adjustment is recommended for any of the evaluated co-administered medications.
Potential for Co-administered Drugs to Influence the Pharmacokinetics of Dulaglutide
In a clinical pharmacology study, the co-administration of a single dose of dulaglutide (1.5 mg) with steady-state sitagliptin (100 mg) caused an increase in dulaglutide AUC and Cmax of approximately 38% and 27%, which is not considered clinically relevant.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
A 2-year carcinogenicity study was conducted with dulaglutide in male and female rats at doses of 0.05, 0.5, 1.5, and 5.0 mg/kg (0.5-, 7-, 20-, and 58-fold the MRHD of 1.5 mg once weekly based on AUC) administered by subcutaneous injection twice weekly. In rats, dulaglutide caused a dose-related and treatment-duration-dependent increase in the incidence of thyroid C-cell tumors (adenomas and/or carcinomas) compared to controls, at ≥7-fold the MRHD based on AUC. A statistically significant increase in C-cell adenomas was observed in rats receiving dulaglutide at ≥0.5 mg/kg. Numerical increases in thyroid C-cell carcinomas occurred at 5 mg/kg (58 times the MRHD based on AUC) and were considered to be treatment-related despite the absence of statistical significance.
A 6-month carcinogenicity study was conducted with dulaglutide in rasH2 transgenic mice at doses of 0.3, 1.0, and 3.0 mg/kg administered by subcutaneous injection twice weekly. Dulaglutide did not produce increased incidences of thyroid C-cell hyperplasia or neoplasia at any dose.
Dulaglutide is a recombinant protein; no genotoxicity studies have been conducted.
Human relevance of thyroid C-cell tumors in rats is unknown and could not be determined by clinical studies or nonclinical studies [see Boxed Warning and Warnings and Precautions (5.1)].
In fertility and early embryonic development studies in male and female rats, no adverse effects of dulaglutide on sperm morphology, mating, fertility, conception, and embryonic survival were observed at up to 16.3 mg/kg (130-fold the MRHD based on AUC). In female rats, an increase in the number of females with prolonged diestrus and a dose-related decrease in the mean number of corpora lutea, implantation sites, and viable embryos were observed at ≥4.9 mg/kg (≥32-fold the MRHD based on AUC), which occurred in the presence of decreased maternal food consumption and body weight gain.
13.2 Animal Toxicology and/or Pharmacology
Zucker diabetic fatty (ZDF) rats were given 0.5, 1.5, or 5.0 mg/kg/twice weekly of dulaglutide (3-, 8-, and 30-fold the MRHD based on AUC) for 3 months. Increases of 12% to 33% in total and pancreatic amylase, but not lipase, were observed at all doses without microscopic pancreatic inflammatory correlates in individual animals. Other changes in the dulaglutide-treated animals included increased interlobular ductal epithelium without active ductal cell proliferation (≥0.5 mg/kg), increased acinar atrophy with/without inflammation (≥1.5 mg/kg), and increased neutrophilic inflammation of the acinar pancreas (5 mg/kg).
Treatment of monkeys for 12 months with 8.15 mg/kg/twice weekly of dulaglutide (nearly 500-fold the MRHD based on AUC) demonstrated no evidence of pancreatic inflammation or pancreatic intraepithelial neoplasia. In 4 of 19 monkeys on dulaglutide treatment, there was an increase in goblet cells within the pancreatic ducts, but no differences from the control group in total amylase or lipase at study termination. There were no proliferative changes in the thyroid C-cells.
-
14 CLINICAL STUDIES
14.1 Glycemic Control Trials in Adults with Type 2 Diabetes Mellitus
TRULICITY has been studied as monotherapy and in combination with metformin, sulfonylurea, metformin and sulfonylurea, metformin and thiazolidinedione, sodium-glucose co-transporter-2 inhibitors (SGLT2i) with or without metformin, basal insulin with or without metformin, and prandial insulin with or without metformin. TRULICITY has also been studied in patients with type 2 diabetes mellitus and moderate to severe renal impairment.
The studies evaluated the use of TRULICITY 0.75 mg and 1.5 mg. Uptitration was not performed in any of the trials; patients were initiated and maintained on either 0.75 mg or 1.5 mg for the duration of the trials. In patients with type 2 diabetes mellitus, TRULICITY produced reductions from baseline in HbA1c compared to placebo. No overall differences in glycemic effectiveness were observed across demographic subgroups (age, gender, race/ethnicity, duration of diabetes).
Monotherapy
In a 52-week double-blind study (26-week primary endpoint), 807 patients inadequately treated with diet and exercise, or with diet and exercise and one antidiabetic agent used at submaximal dose, were randomized to TRULICITY 0.75 mg once weekly, TRULICITY 1.5 mg once weekly, or metformin 1500 to 2000 mg/day following a two-week washout. Seventy-five percent (75%) of the randomized population were treated with one antidiabetic agent at the screening visit. Most patients previously treated with an antidiabetic agent were receiving metformin (~90%) at a median dose of 1000 mg daily and approximately 10% were receiving a sulfonylurea.
Patients had a mean age of 56 years and a mean duration of type 2 diabetes of 3 years. Forty-four percent were male. The White, Black and Asian race accounted for 74%, 7% and 8% of the population, respectively. Twenty-nine percent of the study population were from the US.
Treatment with TRULICITY 0.75 mg and 1.5 mg once weekly resulted in reduction in HbA1c from baseline at the 26-week primary timepoint (Table 3). The difference in observed effect size between TRULICITY 0.75 mg and 1.5 mg, respectively, and metformin excluded the pre-specified non-inferiority margin of 0.4%.
Table 3: Results at Week 26 in a Trial of TRULICITY as Monotherapya Abbreviation: HbA1c = hemoglobin A1c.
a Intent-to-treat population. Last observation carried forward (LOCF) was used to impute missing data. Data post-onset of rescue therapy are treated as missing. At Week 26, primary efficacy was missing for 10%, 12% and 14% of individuals randomized to TRULICITY 0.75 mg, TRULICITY 1.5 mg and metformin, respectively.
b Least-squares mean adjusted for baseline value and other stratification factors.
‡ Subjects included in the analysis are a subset of the ITT population that had at least one post-baseline assessment. The primary analysis included 265 individuals in each of the treatment arms.
26-Week Primary Time Point TRULICITY 0.75 mg TRULICITY 1.5 mg Metformin
1500-2000 mgIntent-to-Treat (ITT) Population (N)‡ 270 269 268 HbA1c (%) (Mean) Baseline 7.6 7.6 7.6 Change from baselineb -0.7 -0.8 -0.6 Fasting Serum Glucose (mg/dL) (Mean) Baseline 161 164 161 Change from baselineb -26 -29 -24 Body Weight (kg) (Mean) Baseline 91.8 92.7 92.4 Change from baselineb -1.4 -2.3 -2.2 Combination Therapy
Add-on to Metformin
In this 104-week placebo-controlled, double-blind study (52-week primary endpoint), 972 patients were randomized to placebo, TRULICITY 0.75 mg once weekly, TRULICITY 1.5 mg once weekly, or sitagliptin 100 mg/day (after 26 weeks, patients in the placebo treatment group received blinded sitagliptin 100 mg/day for the remainder of the study), all as add-on to metformin. Randomization occurred after an 11-week lead-in period to allow for a metformin titration period, followed by a 6-week glycemic stabilization period. Patients had a mean age of 54 years; mean duration of type 2 diabetes of 7 years; 48% were male; race: White, Black and Asian were 53%, 4% and 27%, respectively; and 24% of the study population were in the US.
At the 26-week placebo-controlled time point, the HbA1c change was 0.1%, -1.0%, -1.2%, and -0.6% for placebo, TRULICITY 0.75 mg, TRULICITY 1.5 mg, and sitagliptin, respectively. The percentage of patients who achieved HbA1c <7.0% was 22%, 56%, 62% and 39% for placebo, TRULICITY 0.75 mg, TRULICITY 1.5 mg, and sitagliptin, respectively. At 26 weeks, there was a mean weight reduction of 1.4 kg, 2.7 kg, 3.0 kg, and 1.4 kg for placebo, TRULICITY 0.75 mg, TRULICITY 1.5 mg, and sitagliptin, respectively. There was a mean reduction of fasting glucose of 9 mg/dL, 35 mg/dL, 41 mg/dL, and 18 mg/dL for placebo, TRULICITY 0.75 mg, TRULICITY 1.5 mg, and sitagliptin, respectively.
Treatment with TRULICITY 0.75 mg and 1.5 mg once weekly resulted in a statistically significant reduction in HbA1c compared to placebo (at 26 weeks) and compared to sitagliptin (at 26 and 52 weeks), all in combination with metformin (Table 4 and Figure 3).
Table 4: Results at Week 52 of TRULICITY Compared to Sitagliptin used as Add-On to Metformina Abbreviations: HbA1c = hemoglobin A1c.
a All ITT patients randomized after the dose-finding portion of the study. Last observation carried forward (LOCF) was used to impute missing data. At Week 52 primary efficacy was missing for 15%, 19%, and 20% of individuals randomized to TRULICITY 0.75 mg, TRULICITY 1.5 mg and sitagliptin, respectively.
b Least-squares (LS) mean adjusted for baseline value and other stratification factors.
‡ Subjects included in the analysis are a subset of the ITT population that had at least one post-baseline assessment. The primary analysis included 276, 277, and 270 individuals randomized to TRULICITY 0.75 mg, TRULICITY 1.5 mg and sitagliptin, respectively.
†† Multiplicity adjusted 1-sided p-value <0.001, for superiority of TRULICITY compared to sitagliptin, assessed only for HbA1c.
## p<0.001 TRULICITY compared to sitagliptin, assessed only for HbA1c <7.0%.
52-Week Primary Time Point TRULICITY
0.75 mgTRULICITY
1.5 mgSitagliptin
100 mgIntent-to-Treat (ITT) Population (N)‡ 281 279 273 HbA1c (%) (Mean) Baseline 8.2 8.1 8.0 Change from baselineb -0.9 -1.1 -0.4 Difference from sitagliptinb (95% CI) -0.5 (-0.7, -0.3)†† -0.7 (-0.9, -0.5)†† - Percentage of patients HbA1c <7.0% 49## 59## 33 Fasting Plasma Glucose (mg/dL) (Mean) Baseline 174 173 171 Change from baselineb -30 -41 -14 Difference from sitagliptinb (95% CI) -15 (-22, -9) -27 (-33, -20) - Body Weight (kg) (Mean) Baseline 85.5 86.5 85.8 Change from baselineb -2.7 -3.1 -1.5 Difference from sitagliptinb (95% CI) -1.2 (-1.8, -0.6) -1.5 (-2.1, -0.9) - Add-on to Sulfonylurea
In this 24-week placebo-controlled, double-blind study, 299 patients were randomized to and received placebo or once weekly TRULICITY 1.5 mg, both as add-on to glimepiride. Patients had a mean age of 58 years; mean duration of type 2 diabetes of 8 years; 44% were male; race: White, Black, and Asian were 83%, 4%, and 2%, respectively; and 24% of the study population were in the US.
At 24 weeks, treatment with once weekly TRULICITY 1.5 mg resulted in a statistically significant reduction in HbA1c compared to placebo (Table 5).
Table 5: Results at Week 24 of TRULICITY Compared to Placebo as Add-On to Glimepiridea Abbreviations: HbA1c = hemoglobin A1c.
a Intent-to-treat population. Data post-onset of rescue therapy are treated as missing. At Week 24 primary efficacy was missing for 10% and 12% of individuals randomized to TRULICITY 1.5 mg and placebo, respectively.
b Least-squares mean from ANCOVA adjusted for baseline value and other stratification factors. Placebo multiple imputation, with respect to the baseline values, was used to model a wash-out of the treatment effect for subjects having missing Week 24 data.
c Patients with missing HbA1c data at Week 24 were considered as non-responders.
†† p<0.001 for superiority of TRULICITY 1.5 mg compared to placebo, overall type I error controlled.
24-Week Primary Time Point Placebo TRULICITY
1.5 mgIntent-to-Treat (ITT) Population (N) 60 239 HbA1c (%) (Mean) Baseline 8.4 8.4 Change from baselineb -0.3 -1.3 Difference from placebob (95% CI) -1.1 (-1.4, -0.7)†† Percentage of patients HbA1c <7.0%c 17 50†† Fasting Serum Glucose (mg/dL) (Mean) Baseline 175 178 Change from baselineb 2 -28 Difference from placebob (95% CI) -30 (-44, -15)†† Body Weight (kg) (Mean) Baseline 89.5 84.5 Change from baselineb -0.2 -0.5 Difference from placebob (95% CI) -0.4 (-1.2, 0.5) Add-on to Metformin and Thiazolidinedione
In this 52-week placebo-controlled study (26-week primary endpoint), 976 patients were randomized to and received placebo, TRULICITY 0.75 mg once weekly, TRULICITY 1.5 mg once weekly, or exenatide 10 mcg BID, all as add-on to maximally tolerated doses of metformin (≥1500 mg per day) and pioglitazone (up to 45 mg per day). Exenatide treatment group assignment was open-label while the treatment assignments to placebo, TRULICITY 0.75 mg, and TRULICITY 1.5 mg were blinded. After 26 weeks, patients in the placebo treatment group were randomized to either TRULICITY 0.75 mg once weekly or TRULICITY 1.5 mg once weekly to maintain study blind. Randomization occurred after a 12-week lead-in period; during the initial 4 weeks of the lead-in period, patients were titrated to maximally tolerated doses of metformin and pioglitazone; this was followed by an 8-week glycemic stabilization period prior to randomization. Patients randomized to exenatide started at a dose of 5 mcg BID for 4 weeks and then were escalated to 10 mcg BID. Patients had a mean age of 56 years; mean duration of type 2 diabetes of 9 years; 58% were male; race: White, Black and Asian were 74%, 8% and 3%, respectively; and 81% of the study population were in the US.
Treatment with TRULICITY 0.75 mg and 1.5 mg once weekly resulted in a statistically significant reduction in HbA1c compared to placebo (at 26 weeks) and compared to exenatide at 26 weeks (Table 6 and Figure 4). Over the 52-week study period, the percentage of patients who required glycemic rescue was 8.9% in the TRULICITY 0.75 mg once weekly + metformin and pioglitazone treatment group, 3.2% in the TRULICITY 1.5 mg once weekly + metformin and pioglitazone treatment group, and 8.7% in the exenatide BID + metformin and pioglitazone treatment group.
Table 6: Results at Week 26 of TRULICITY Compared to Placebo and Exenatide, All as Add-On to Metformin and Thiazolidinedionea Abbreviations: BID = twice daily; HbA1c = hemoglobin A1c.
a Intent-to-treat population. Last observation carried forward (LOCF) was used to impute missing data. Data post-onset of rescue therapy are treated as missing. At Week 26, primary efficacy was missing for 23%, 10%, 7% and 12% of individuals randomized to placebo, TRULICITY 0.75 mg, TRULICITY 1.5 mg, and exenatide, respectively.
b Least-squares (LS) mean adjusted for baseline value and other stratification factors.
‡ Subjects included in the analysis are a subset of the ITT population that had at least one post-baseline assessment. The primary analysis included 119, 269, 271 and 266 individuals randomized to placebo, TRULICITY 0.75 mg, TRULICITY 1.5 mg, and exenatide, respectively.
‡‡ Multiplicity adjusted 1-sided p-value <0.001, for superiority of TRULICITY compared to placebo, assessed only for HbA1c.
†† Multiplicity adjusted 1-sided p-value <0.001, for superiority of TRULICITY compared to exenatide, assessed only for HbA1c.
** p<0.001 TRULICITY compared to placebo, assessed only for HbA1c <7.0%.
## p<0.001 TRULICITY compared to exenatide, assessed only for HbA1c <7.0%.
26-Week Primary Time Point Placebo TRULICITY
0.75 mgTRULICITY
1.5 mgExenatide
10 mcg BIDIntent-to-Treat (ITT) Population (N)‡ 141 280 279 276 HbA1c (%) (Mean) Baseline 8.1 8.1 8.1 8.1 Change from baselineb -0.5 -1.3 -1.5 -1.0 Difference from placebob (95% CI) - -0.8 (-1.0, -0.7)‡‡ -1.1 (-1.2, -0.9)‡‡ - Difference from exenatideb (95% CI) - -0.3 (-0.4, -0.2)†† -0.5 (-0.7, -0.4)†† - Percentage of patients HbA1c <7.0% 43 66**, ## 78**, ## 52 Fasting Serum Glucose (mg/dL) (Mean) Baseline 166 159 162 164 Change from baselineb -5 -34 -42 -24 Difference from placebob (95% CI) - -30 (-36, -23) -38 (-45, -31) - Difference from exenatideb (95% CI) - -10 (-15, -5) -18 (-24, -13) - Body Weight (kg) (Mean) Baseline 94.1 95.5 96.2 97.4 Change from baselineb 1.2 0.2 -1.3 -1.1 Difference from placebob (95% CI) - -1.0 (-1.8, -0.3) -2.5 (-3.3, -1.8) - Difference from exenatideb (95% CI) - 1.3 (0.6, 1.9) -0.2 (-0.9, 0.4) - Combination Therapy with SGLT2i, with or without Metformin
In this 24-week placebo-controlled, double-blind study, 423 patients were randomized to and received TRULICITY 0.75 mg, TRULICITY 1.5 mg, or placebo, as add-on to sodium-glucose co-transporter 2 inhibitor (SGLT2i) therapy (96% with and 4% without metformin). Trulicity was administered once weekly, and SGLT2i was administered according to the local country label. Patients had a mean age of 57 years; mean duration of type 2 diabetes of 9.4 years; 50% were male; race: White, Black, and Asian were 89%, 3%, and 0.2%, respectively; and 21% of the study population was in the US.
At 24 weeks, treatment with once weekly TRULICITY 0.75 mg and 1.5 mg resulted in a statistically significant reduction from baseline in HbA1c compared to placebo (Table 7).
The mean baseline body weight was 90.5, 91.1, and 92.9 kg in the placebo, TRULICITY 0.75 mg, and TRULICITY 1.5 mg groups, respectively. The mean changes from baseline in body weight at Week 24 were -2.0, -2.5, and -2.9 kg for placebo, TRULICITY 0.75 mg, and TRULICITY 1.5 mg, respectively. The difference from placebo (95% CI) was -0.9 kg (-1.7, -0.1) for TRULICITY 1.5 mg.
Table 7: Results at Week 24 of TRULICITY as Add-on to SGLT2ia Abbreviations: HbA1c = hemoglobin A1c; SGLT2i = sodium-glucose co-transporter-2 inhibitors.
a Intent-to-treat population. At Week 24, primary efficacy was missing for 3%, 4%, and 6% of individuals treated with placebo, TRULICITY 0.75 mg, and TRULICITY 1.5 mg, respectively.
b Least-squares mean adjusted for baseline value and other stratification factors. Placebo multiple imputation, using baseline and 24-week values from the placebo arm, was applied to model a washout of the treatment effect for patients missing 24-week values (HbA1c, fasting serum glucose, and body weight).
c Patients with missing HbA1c data at Week 24 were considered as non-responders.
†† p<0.001 for superiority of TRULICITY compared to placebo, overall type I error controlled.
24-Week Primary Time Point Placebo TRULICITY
0.75 mgTRULICITY
1.5 mgIntent-to-Treat (ITT) Population (N) 140 141 142 HbA1c (%) (Mean) Baseline 8.1 8.1 8.0 Change from baselineb -0.6 -1.2 -1.3 Difference from placebob (95% CI) - -0.7 (-0.8, -0.5)†† -0.8 (-0.9, -0.6)†† Percentage of patients HbA1c <7.0%c 31 59†† 67†† Fasting Serum Glucose (mg/dL) (Mean) Baseline 153 162 161 Change from baselineb -6 -25 -30 Difference from placebob (95% CI) - -19 (-25, -13) -24 (-30, -18)†† Add-on to Metformin and Sulfonylurea
In this 78-week (52-week primary endpoint) open-label comparator study (double-blind with respect to TRULICITY dose assignment), 807 patients were randomized to and received TRULICITY 0.75 mg once weekly, TRULICITY 1.5 mg once weekly, or insulin glargine once daily, all as add-on to maximally tolerated doses of metformin and glimepiride. Randomization occurred after a 10-week lead-in period; during the initial 2 weeks of the lead-in period, patients were titrated to maximally tolerated doses of metformin and glimepiride. This was followed by a 6- to 8-week glycemic stabilization period prior to randomization.
Patients randomized to insulin glargine were started on a dose of 10 units once daily. Insulin glargine dose adjustments occurred twice weekly for the first 4 weeks of treatment based on self-measured fasting plasma glucose (FPG), followed by once weekly titration through Week 8 of study treatment, utilizing an algorithm that targeted a fasting plasma glucose of <100 mg/dL. Only 24% of patients were titrated to goal at the 52-week primary endpoint. The dose of glimepiride could be reduced or discontinued after randomization (at the discretion of the investigator) in the event of persistent hypoglycemia. The dose of glimepiride was reduced or discontinued in 28%, 32%, and 29% of patients randomized to TRULICITY 0.75 mg, TRULICITY 1.5 mg, and glargine.
Patients had a mean age of 57 years; mean duration of type 2 diabetes of 9 years; 51% were male; race: White, Black and Asian were 71%, 1% and 17%, respectively; and 0% of the study population were in the US.
Treatment with TRULICITY once weekly resulted in a reduction in HbA1c from baseline at 52 weeks when used in combination with metformin and sulfonylurea (Table 8). The difference in observed effect size between TRULICITY 0.75 mg and 1.5 mg, respectively, and glargine in this trial excluded the pre-specified non-inferiority margin of 0.4%.
Table 8: Results at Week 52 of TRULICITY Compared to Insulin Glargine, Both as Add-on to Metformin and Sulfonylureaa Abbreviations: HbA1c = hemoglobin A1c.
a Intent-to-treat population. Last observation carried forward (LOCF) was used to impute missing data. Data post-onset of rescue therapy are treated as missing. At Week 52, primary efficacy was missing for 17%, 13% and 12% of individuals randomized to TRULICITY 0.75 mg, TRULICITY 1.5 mg and glargine, respectively.
b Least-squares (LS) mean adjusted for baseline value and other stratification factors.
‡ Subjects included in the analysis are a subset of the ITT population that had at least one post-baseline assessment. The primary analysis included 267, 263 and 259 individuals randomized to TRULICITY 0.75 mg, TRULICITY 1.5 mg, and glargine, respectively.
52-Week Primary Time Point TRULICITY
0.75 mgTRULICITY
1.5 mgInsulin Glargine Intent-to-Treat (ITT) Population (N)‡ 272 273 262 HbA1c (%) (Mean) Baseline 8.1 8.2 8.1 Change from baselineb -0.8 -1.1 -0.6 Fasting Serum Glucose (mg/dL) (Mean) Baseline 161 165 163 Change from baselineb -16 -27 -32 Difference from insulin glargineb (95% CI) 16 (9, 23) 5 (-2, 12) - Body Weight (kg) (Mean) Baseline 86.4 85.2 87.6 Change from baselineb -1.3 -1.9 1.4 Difference from insulinb (95% CI) -2.8 (-3.4, -2.2) -3.3 (-3.9, -2.7) - Combination Therapy with Basal Insulin, with or without Metformin
In this 28-week placebo-controlled, double-blind study, 300 patients were randomized to placebo or once weekly TRULICITY 1.5 mg, as add-on to titrated basal insulin glargine (with or without metformin). Patients had a mean age of 60 years; mean duration of type 2 diabetes of 13 years; 58% were male; race: White, Black, and Asian were 94%, 4%, and 0.3%, respectively; and 20% of the study population was in the US.
The mean starting dose of insulin glargine was 37 units/day for patients receiving placebo and 41 units/day for patients receiving TRULICITY 1.5 mg. At randomization, the initial insulin glargine dose in patients with HbA1c <8.0% was reduced by 20%.
At 28 weeks, treatment with once weekly TRULICITY 1.5 mg resulted in a statistically significant reduction in HbA1c compared to placebo (Table 9).
Table 9: Results at Week 28 of TRULICITY Compared to Placebo as Add-On to Basal Insulina Abbreviations: HbA1c = hemoglobin A1c.
a Intent-to-treat population. At Week 28, primary efficacy was missing for 12% and 8% of individuals randomized to placebo and TRULICITY 1.5 mg, respectively.
b Least-squares mean from ANCOVA adjusted for baseline value and other stratification factors. Placebo multiple imputation, with respect to baseline values, was used to model a wash-out of the treatment effect for subjects having missing Week 28 data.
c Patients with missing HbA1c data at Week 28 were considered as non-responders.
†† p<0.001 for superiority of TRULICITY 1.5 mg compared to placebo, overall type I error controlled.
† p≤0.005 for superiority of TRULICITY 1.5 mg compared to placebo, overall type I error controlled.
28-Week Primary Time Point Placebo TRULICITY
1.5 mgIntent-to-Treat (ITT) Population (N) 150 150 HbA1c (%) (Mean) Baseline 8.3 8.4 Change from baselineb -0.7 -1.4 Difference from placebob (95% CI) -0.7 (-0.9, -0.5)†† Percentage of patients HbA1c <7.0%c 33 67†† Fasting Serum Glucose (mg/dL) (Mean) Baseline 156 157 Change from baselineb -30 -44 Difference from placebob (95% CI) -14 (-23, -4)† Body Weight (kg) (Mean) Baseline 92.6 93.3 Change from baselineb 0.8 -1.3 Difference from placebob (95% CI) -2.1 (-2.9, -1.4)†† Combination Therapy with Prandial Insulin, with or without Metformin
In this 52-week (26-week primary endpoint) open-label comparator study (double-blind with respect to TRULICITY dose assignment), 884 patients on 1 or 2 insulin injections per day were enrolled. Randomization occurred after a 9-week lead-in period; during the initial 2 weeks of the lead-in period, patients continued their pre-study insulin regimen but could be initiated and/or up-titrated on metformin, based on investigator discretion; this was followed by a 7-week glycemic stabilization period prior to randomization.
At randomization, patients discontinued their pre-study insulin regimen and were randomized to TRULICITY 0.75 mg once weekly, TRULICITY 1.5 mg once weekly, or insulin glargine once daily, all in combination with prandial insulin lispro 3 times daily, with or without metformin. Insulin lispro was titrated in each arm based on preprandial and bedtime glucose, and insulin glargine was titrated to a fasting plasma glucose goal of <100 mg/dL. Only 36% of patients randomized to glargine were titrated to the fasting glucose goal at the 26-week primary timepoint.
Patients had a mean age of 59 years; mean duration of type 2 diabetes of 13 years; 54% were male; race: White, Black and Asian were 79%, 10% and 4%, respectively; and 33% of the study population were in the US.
Treatment with TRULICITY 0.75 mg and 1.5 mg once weekly resulted in a reduction in HbA1c from baseline. The difference in observed effect size between TRULICITY 0.75 mg and 1.5 mg, respectively, and glargine in this trial excluded the pre-specified non-inferiority margin of 0.4%.
Table 10: Results at Week 26 of TRULICITY Compared to Insulin Glargine, Both in Combination with Insulin Lisproa Abbreviation: HbA1c = hemoglobin A1c
a Intent-to-treat population. Last observation carried forward (LOCF) was used to impute missing data. Data post-onset of rescue therapy are treated as missing. At Week 26, primary efficacy was missing for 14%, 15%, and 14% of individuals randomized to TRULICITY 0.75 mg, TRULICITY 1.5 mg and glargine, respectively.
b Least-squares (LS) mean adjusted for baseline value and other stratification factors.
‡ Subjects included in the analysis are a subset of the ITT population that had at least one post-baseline assessment. The primary analysis included 275, 273 and 276 individuals randomized to TRULICITY 0.75 mg, TRULICITY 1.5 mg, and glargine, respectively.
26-Week Primary Time Point TRULICITY
0.75 mgTRULICITY
1.5 mgInsulin Glargine Intent-to-Treat (ITT) Population (N)‡ 293 295 296 HbA1c (%) (Mean) Baseline 8.4 8.5 8.5 Change from baselineb -1.6 -1.6 -1.4 Fasting Serum Glucose (mg/dL) (Mean) Baseline 150 157 154 Change from baselineb 4 -5 -28 Difference from insulin glargineb (95% CI) 32 (24, 41) 24 (15, 32) - Body Weight (kg) (Mean) Baseline 91.7 91.0 90.8 Change from baselineb 0.2 -0.9 2.3 Difference from insulin glargineb (95% CI) -2.2 (-2.8, -1.5) -3.2 (-3.8, -2.6) - Moderate to Severe Chronic Kidney Disease
In this 52-week (26-week primary endpoint) open-label comparator study (double-blind with respect to TRULICITY dose assignment), a total of 576 patients with type 2 diabetes were randomized and treated to compare TRULICITY 0.75 mg and 1.5 mg with insulin glargine (NCT01621178).
Patients on insulin and other antidiabetic therapy (e.g., oral antidiabetic drugs, pramlintide) had non-insulin therapies discontinued and had their insulin dose adjusted for 12 weeks prior to randomization. Patients on insulin therapy alone maintained a stable insulin dose for 3 weeks prior to randomization. At randomization, patients discontinued their pre-study insulin regimen and patients were randomized to TRULICITY 0.75 mg once weekly, TRULICITY 1.5 mg once weekly, or insulin glargine once daily, all in combination with prandial insulin lispro. For patients randomized to insulin glargine, the initial insulin glargine dose was based on the basal insulin dose prior to randomization. Insulin glargine was allowed to be titrated with a fasting plasma glucose goal of ≤150 mg/dL. Insulin lispro was allowed to be titrated with a preprandial and bedtime glucose goal of ≤180 mg/dL.
Patients had a mean age of 65 years; a mean duration of type 2 diabetes of 18 years; 52% were male; race: White, Black, and Asian were 69%, 16%, and 3%, respectively; and 32% of the study population were in the US. At baseline, overall mean eGFR was 38 mL/min/1.73 m2, 30% of patients had eGFR <30 mL/min/1.73 m2, and 45% of patients had macroalbuminuria. Patients on over 70 units/day of basal insulin were excluded from the study.
Treatment with TRULICITY 0.75 mg and 1.5 mg once weekly resulted in a reduction in HbA1c at 26-weeks from baseline. The difference in observed effect size between TRULICITY 0.75 mg and 1.5 mg, respectively, and glargine in this trial excluded the pre-specified non-inferiority margin of 0.4%. Mean fasting plasma glucose increased in the TRULICITY arms (Table 11).
Mean baseline body weight was 90.9 kg, 88.1 kg, and 88.2 kg in the TRULICITY 0.75 mg, TRULICITY 1.5 mg, and insulin glargine arms, respectively. The mean changes from baseline at Week 26 were -1.1, -2, and 1.9 kg in the TRULICITY 0.75 mg, TRULICITY 1.5 mg, and insulin glargine arms, respectively.
Table 11: Results at Week 26 of TRULICITY Compared to Insulin Glargine, Both in Combination with Insulin Lispro, in Patients with Moderate to Severe Chronic Kidney Diseasea Abbreviation: HbA1c = hemoglobin A1c
a Intent-to-treat population (all randomized and treated subjects) was used in the analysis regardless of discontinuation of study drug or initiation of rescue therapy. At Week 26, primary efficacy was missing for 12%, 15%, and 9% of individuals randomized to and treated with TRULICITY 0.75 mg, TRULICITY 1.5 mg, and insulin glargine, respectively. Missing data were imputed using multiple imputation within treatment group.
b Least-squares (LS) mean from ANCOVA pattern mixture model adjusted for baseline value and other stratification factors.
26-Week Primary Time Point TRULICITY
0.75 mgTRULICITY
1.5 mgInsulin Glargine Intent-to-Treat Population (N) 190 192 194 HbA1c (%) (Mean) Baseline 8.6 8.6 8.6 Change from baselineb -0.9 -1.0 -1.0 Difference from insulin glargineb (95% CI) 0.0 (-0.2, 0.3) -0.1 (-0.3, 0.2) Percentage of patients HbA1c <8.0% 73 75 74 Fasting Serum Glucose (mg/dL) (Mean) Baseline 167 161 170 Change from baselineb 6 14 -23 Difference from insulin glargineb (95% CI) 30 (16, 43) 37 (24, 50) 14.2 Cardiovascular Outcomes Trial in Adults with Type 2 Diabetes Mellitus and Cardiovascular Disease or Multiple Cardiovascular Risk Factors
The REWIND trial (NCT01394952) was a multi-national, multi-center, randomized, placebo-controlled, double-blind trial. In this study, 9901 adult patients with type 2 diabetes mellitus and established cardiovascular (CV) disease or multiple cardiovascular risk factors were randomized to TRULICITY 1.5 mg or placebo both added to standard of care. The median follow-up duration was 5.4 years. The primary endpoint was the time to the first occurrence of a composite 3-component Major Adverse Cardiovascular Events (MACE) outcome, which included CV death, non-fatal myocardial infarction (MI), and non-fatal stroke.
Patients eligible to enter the trial were 50 years of age or older who had type 2 diabetes mellitus, had an HbA1c value ≤9.5% with no lower limit at screening, and had either established CV disease, or did not have established CV disease but had multiple CV risk factors. Patients who were confirmed to have established CV disease (31.5% of randomized patients) had a history of at least one of the following: MI (16.2%); myocardial ischemia by a stress test or with cardiac imaging (9.3%); ischemic stroke (5.3%); coronary, carotid, or peripheral artery revascularization (18.0%); unstable angina (5.9%); or hospitalization for unstable angina with at least one of the following: ECG changes, myocardial ischemia on imaging, or a need for percutaneous coronary intervention (12.0%). Patients confirmed to be without established CV disease, but with multiple CV risk factors, comprised 62.8% of the randomized trial population.
At baseline, demographic and disease characteristics were balanced between treatment groups. Patients had a mean age of 66 years; 46% were female; race: White, Black, and Asian were 76%, 7%, and 4%, respectively.
The median baseline HbA1c was 7.2%. The mean duration of type 2 diabetes was 10.5 years and the mean BMI was 32.3 kg/m2.
At baseline, 50.5% of patients had mild renal impairment (eGFR ≥60 but <90 mL/min/1.73m2), 21.6% had moderate renal impairment (eGFR ≥30 but <60 mL/min/1.73m2), and 1.1% of patients had severe renal impairment (eGFR <30 mL/min/1.73m2) out of 9713 patients whose eGFR were available.
At baseline, 94.7% of patients were taking antidiabetic medication, with 10.5% of patients taking three or more antidiabetic drugs. The most common background antidiabetic drugs used at baseline were metformin (81.2%), sulfonylurea (46.0%), and insulin (23.9%). At baseline, CV disease and risk factors were managed with ACE inhibitors or angiotensin receptor blockers (81.5%), beta blockers (45.6%), calcium channel blockers (34.4%), diuretics (46.5%), statin therapy (66.1%), antithrombotic agents (58.7%), and aspirin (51.7%). During the trial, investigators were to modify anti-diabetic and cardiovascular medications to achieve local standard of care treatment targets with respect to blood glucose, lipids, and blood pressure, and manage patients recovering from an acute coronary syndrome or stroke event per local treatment guidelines.
For the primary analysis, a Cox proportional hazards model was used to test for superiority. Type I error was controlled across multiple tests. TRULICITY significantly reduced the risk of first occurrence of primary composite endpoint of CV death, non-fatal MI, or non-fatal stroke (HR: 0.88, 95% CI 0.79, 0.99). Refer to Figure 5 and Table 12.
Vital status was available for 99.7% of subjects in the trial. A total of 1128 deaths were recorded during the REWIND trial. A majority of the deaths in the trial were adjudicated as CV deaths, and non-CV deaths were comparable between the treatment groups (4.4% in patients treated with TRULICITY and 5.0% in patients treated with placebo). There were 536 all-cause deaths (10.8%) in the dulaglutide group compared to 592 deaths (12.0%) in the placebo group.
Table 12: Treatment Effect for MACE and the Individual Components in the REWIND Trial, Median Study Observation Time of 5.4 yearsa a All randomized patients.
b Cox-proportional hazards model with treatment as a factor. Type I error was controlled for the primary and secondary endpoints.
c p=0.026 for superiority (2-sided).
d Number and percentage of patients with events.
e Results for components of MACE, fatal and non-fatal stroke, and fatal and non-fatal MI are listed descriptively for supportive purposes. No statistical significance should be inferred since these CIs are not adjusted for multiplicity.
Time to First Occurrence of: TRULICITY
N=4949Placebo
N=4952Hazard Ratio
(95% CI)bComposite of non-fatal myocardial infarction, non-fatal stroke, cardiovascular death (MACE)d 594 (12.0%) 663 (13.4%) 0.88 (0.79, 0.99)c Cardiovascular deathd,e 317 (6.4%) 346 (7.0%) 0.91 (0.78, 1.06) Non-fatal myocardial infarctiond,e 205 (4.1%) 212 (4.3%) 0.96 (0.79, 1.16) Non-fatal stroked,e 135 (2.7%) 175 (3.5%) 0.76 (0.61, 0.95) Fatal or non-fatal myocardial infarctiond,e 223 (4.5%) 231 (4.7%) 0.96 (0.79, 1.15) Fatal or non-fatal stroked,e 158 (3.2%) 205 (4.1%) 0.76 (0.62, 0.94) -
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Each TRULICITY single-dose pen is packaged in a cardboard outer carton.
Carton of 4 Single-Dose Pens
- 0.75 mg/0.5 mL solution in a single-dose pen (NDC: 0002-1433-80)
- 1.5 mg/0.5 mL solution in a single-dose pen (NDC: 0002-1434-80)
16.2 Storage and Handling
- Store TRULICITY in the refrigerator at 36°F to 46°F (2°C to 8°C). Do not use TRULICITY beyond the expiration date.
- If needed, each single-dose pen can be kept at room temperature, not to exceed 86°F (30°C) for a total of 14 days.
- Do not freeze TRULICITY. Do not use TRULICITY if it has been frozen.
- TRULICITY must be protected from light. Storage of TRULICITY in the original carton is recommended until time of administration.
- Discard the TRULICITY single-dose pen after use in a puncture-resistant container.
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved Medication Guide
- Inform patients that TRULICITY causes benign and malignant thyroid C-cell tumors in rats and that the human relevance of this finding has not been determined. Counsel patients to report symptoms of thyroid tumors (e.g., a lump in the neck, persistent hoarseness, dysphagia, or dyspnea) to their physician [see Boxed Warning and Warnings and Precautions (5.1)].
- Inform patients that persistent severe abdominal pain, that may radiate to the back and which may (or may not) be accompanied by vomiting, is the hallmark symptom of acute pancreatitis. Instruct patients to discontinue TRULICITY promptly, and to contact their physician, if persistent severe abdominal pain occurs [see Warnings and Precautions (5.2)].
- The risk of hypoglycemia may be increased when TRULICITY is used in combination with a medicine that can cause hypoglycemia, such as a sulfonylurea or insulin. Review and reinforce instructions for hypoglycemia management when initiating TRULICITY therapy, particularly when concomitantly administered with a sulfonylurea or insulin [see Warnings and Precautions (5.3)].
- Advise patients of the potential risk of dehydration due to gastrointestinal adverse reactions and take precautions to avoid fluid depletion. Inform patients treated with TRULICITY of the potential risk for worsening renal function and explain the associated signs and symptoms of renal impairment, as well as the possibility of dialysis as a medical intervention if renal failure occurs [see Warnings and Precautions (5.5)].
- Inform patients that serious hypersensitivity reactions have been reported with use of TRULICITY. Advise patients on the symptoms of hypersensitivity reactions and instruct them to stop taking TRULICITY and seek medical advice promptly if such symptoms occur [see Warnings and Precautions (5.4)].
- Inform patients to contact their physician if changes in vision are experienced during treatment with TRULICITY [see Warnings and Precautions (5.7)].
- Advise women to inform their healthcare provider if they are pregnant or intend to become pregnant [see Use in Specific Populations (8.1)].
- Prior to initiation of TRULICITY, train patients on proper injection technique to ensure a full dose is delivered. Refer to the accompanying Instructions for Use for complete administration instructions with illustrations [see Dosage and Administration (2)].
- Inform patients of the potential risks and benefits of TRULICITY and of alternative modes of therapy. Inform patients about the importance of adherence to dietary instructions, regular physical activity, periodic blood glucose monitoring and HbA1c testing, recognition and management of hypoglycemia and hyperglycemia, and assessment for diabetes complications. During periods of stress such as fever, trauma, infection, or surgery, medication requirements may change and advise patients to seek medical advice promptly.
- Each weekly dose of TRULICITY can be administered at any time of day, with or without food. The day of once weekly administration can be changed if necessary, as long as the last dose was administered 3 or more days before. If a dose is missed and there are at least 3 days (72 hours) until the next scheduled dose, it should be administered as soon as possible. Thereafter, patients can resume their usual once weekly dosing schedule. If a dose is missed and the next regularly scheduled dose is due in 1 or 2 days, the patient should not administer the missed dose and instead resume TRULICITY with the next regularly scheduled dose [see Dosage and Administration (2)].
- Advise patients treated with TRULICITY of the potential risk of gastrointestinal side effects [see Adverse Reactions (6.1)].
- Instruct patients to read the Medication Guide and the Instructions for Use before starting TRULICITY therapy and review them each time the prescription is refilled. Instruct patients to inform their doctor or pharmacist if they develop any unusual symptom, or if any known symptom persists or worsens.
- Inform patients that response to all diabetic therapies should be monitored by periodic measurements of blood glucose and HbA1c levels, with a goal of decreasing these levels towards the normal range. HbA1c is especially useful for evaluating long-term glycemic control.
-
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration
TRU-0005-MG-20200221Revised: February 2020
Medication Guide
TRULICITY® (TRU-li-si-tee) (dulaglutide)
injection, for subcutaneous useRead this Medication Guide before you start using TRULICITY and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment. What is the most important information I should know about TRULICITY?
TRULICITY may cause serious side effects, including:
- Possible thyroid tumors, including cancer. Tell your healthcare provider if you get a lump or swelling in your neck, hoarseness, trouble swallowing, or shortness of breath. These may be symptoms of thyroid cancer. In studies with rats or mice, TRULICITY and medicines that work like TRULICITY caused thyroid tumors, including thyroid cancer. It is not known if TRULICITY will cause thyroid tumors or a type of thyroid cancer called medullary thyroid carcinoma (MTC) in people.
- Do not use TRULICITY if you or any of your family have ever had a type of thyroid cancer called medullary thyroid carcinoma (MTC), or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
What is TRULICITY? - TRULICITY is an injectable prescription medicine that is used:
- along with diet and exercise to improve blood sugar (glucose) in adults with type 2 diabetes mellitus.
- to reduce the risk of major cardiovascular events such as death, heart attack, or stroke in adults with type 2 diabetes mellitus with known heart disease or multiple cardiovascular risk factors.
- It is not known if TRULICITY can be used in people who have had pancreatitis.
- TRULICITY is not a substitute for insulin and is not for use in people with type 1 diabetes or people with diabetic ketoacidosis.
- TRULICITY is not recommended for use in people with severe stomach or intestinal problems.
- It is not known if TRULICITY is safe and effective for use in children. TRULICITY should not be used in children under 18 years of age.
Do not use TRULICITY if:
- you or any of your family have ever had a type of thyroid cancer called medullary thyroid carcinoma (MTC) or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
- you are allergic to dulaglutide or any of the ingredients in TRULICITY. See "What are the possible side effects of TRULICITY?" See the end of this Medication Guide for a complete list of ingredients in TRULICITY.
Before using TRULICITY, tell your healthcare provider if you have any medical conditions including:
- have or have had problems with your pancreas, kidneys or liver.
- have severe problems with your stomach, such as slowed emptying of your stomach (gastroparesis) or problems with digesting food.
- have a history of diabetic retinopathy.
- are pregnant or plan to become pregnant. It is not known if TRULICITY will harm your unborn baby. Tell your healthcare provider if you become pregnant while using TRULICITY.
- are breastfeeding or plan to breastfeed. It is not known if TRULICITY passes into your breast milk. You and your healthcare provider should decide if you should breastfeed while taking TRULICITY.
Before using TRULICITY, talk to your healthcare provider about low blood sugar and how to manage it. Tell your healthcare provider if you are taking other medicines to treat diabetes including insulin or sulfonylureas.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.How should I use TRULICITY?
- Read the Instructions for Use that comes with TRULICITY.
- Use TRULICITY exactly as your healthcare provider tells you to.
- Your healthcare provider should show you how to use TRULICITY before you use it for the first time.
- TRULICITY is injected under the skin (subcutaneously) of your stomach (abdomen), thigh, or upper arm. Do not inject TRULICITY into a muscle (intramuscularly) or vein (intravenously).
- Use TRULICITY 1 time each week on the same day each week at any time of the day.
- You may change the day of the week as long as your last dose was given 3 or more days before.
- If you miss a dose of TRULICITY, take the missed dose as soon as possible if there are at least 3 days (72 hours) until your next scheduled dose. If there are less than 3 days remaining, skip the missed dose and take your next dose on the regularly scheduled day. Do not take 2 doses of TRULICITY within 3 days of each other.
- TRULICITY may be taken with or without food.
- Do not mix insulin and TRULICITY together in the same injection.
- You may give an injection of TRULICITY and insulin in the same body area (such as, your stomach area), but not right next to each other.
- Change (rotate) your injection site with each weekly injection. Do not use the same site for each injection.
- If you take too much TRULICITY, call your healthcare provider or go to the nearest emergency room right away.
- Do not share your TRULICITY pen with another person. You may give another person an infection or get an infection from them.
- Change in level of physical activity or exercise, weight gain or loss, increased stress, illness, change in diet, or because of other medicines you take.
What are the possible side effects of TRULICITY?
TRULICITY may cause serious side effects, including:
- See “What is the most important information I should know about TRULICITY?”
- Inflammation of your pancreas (pancreatitis). Stop using TRULICITY and call your healthcare provider right away if you have severe pain in your stomach area (abdomen) that will not go away, with or without vomiting. You may feel the pain from your abdomen to your back.
-
Low blood sugar (hypoglycemia). Your risk for getting low blood sugar may be higher if you use TRULICITY with another medicine that can cause low blood sugar, such as a sulfonylurea or insulin.
- Signs and symptoms of low blood sugar may include:
- dizziness or light-headedness
- sweating
- confusion or drowsiness
- headache
- blurred vision
- slurred speech
- shakiness
- fast heartbeat
- anxiety, irritability, or mood changes
- hunger
- weakness
- feeling jittery
- Serious allergic reactions. Stop using TRULICITY and get medical help right away if you have any symptoms of a serious allergic reaction including:
- swelling of your face, lips, tongue or throat
- problems breathing or swallowing
- severe rash or itching
- fainting or feeling dizzy
- very rapid heartbeat
- Kidney problems (kidney failure). In people who have kidney problems, diarrhea, nausea, and vomiting may cause a loss of fluids (dehydration) which may cause kidney problems to get worse.
- Severe stomach problems. Other medicines like TRULICITY may cause severe stomach problems. It is not known if TRULICITY causes or worsens stomach problems.
- Changes in vision. Tell your healthcare provider if you have changes in vision during treatment with TRULICITY.
- nausea
- diarrhea
- vomiting
- stomach (abdominal) pain
- decreased appetite
Talk to your healthcare provider about any side effect that bothers you or does not go away. These are not all the possible side effects of TRULICITY.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.General information about the safe and effective use of TRULICITY.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use TRULICITY for a condition for which it was not prescribed. Do not give TRULICITY to other people, even if they have the same symptoms you have. It may harm them.
This Medication Guide summarizes the most important information about TRULICITY. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about TRULICITY that is written for health professionals.What are the ingredients in TRULICITY?
Active ingredient: dulaglutide
Inactive ingredients: citric acid anhydrous, mannitol, polysorbate 80, trisodium citrate dihydrate, in water for injection
TRULICITY® is a registered trademark of Eli Lilly and Company.
Manufactured by: Eli Lilly and Company, Indianapolis, IN 46285, USA, US License Number 1891
www.TRULICITY.com.
Copyright © 2014, 2020 Eli Lilly and Company. All rights reserved.
For more information, go to www.TRULICITY.com or call 1-800-545-5979. -
INSTRUCTIONS FOR USE
-
INSTRUCTIONS FOR USE
Storage and Handling - Store your Pen in the refrigerator between 36°F to 46°F (2°C to 8°C).
- You may store your Pen at room temperature below 86°F (30°C) for up to a total of 14 days.
- Do not freeze your Pen. If the Pen has been frozen, throw the Pen away and use a new Pen.
- Storage of your Pen in the original carton is recommended. Protect your Pen from direct heat and light.
- The Pen has glass parts. Handle it carefully. If you drop it on a hard surface, do not use it. Use a new Pen for your injection.
- Keep your TRULICITY Pen and all medicines out of the reach of children.
-
PACKAGE LABEL – Trulicity®, 0.75 mg/0.5 mL, Single-Dose Pens
NDC: 0002-1433-80
4 Single-Dose Pens
Each pen delivers a 0.75 mg dose.
Use one pen every week.
trulilcity®
(dulaglutide) injection
0.75 mg/0.5 mL
once weekly
Rx only
For subcutaneous use only
Single-Dose Only
Dispense the accompanying Medication Guide to each patient.
www.trulicity.com
Lilly
-
PACKAGE LABEL – Trulicity®, 1.5 mg/0.5 mL, Single-Dose Pens
NDC: 0002-1434-80
4 Single-Dose Pens
Each pen delivers a 1.5 mg dose.
Use one pen every week.
trulicity®
(dulaglutide) injection
1.5 mg/0.5 mL
once weekly
Rx only
For subcutaneous use only
Single-Dose Only
Dispense the accompanying Medication Guide to each patient.
www.trulicity.com
Lilly
-
INGREDIENTS AND APPEARANCE
TRULICITY
dulaglutide injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0002-1433 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Dulaglutide (UNII: WTT295HSY5) (Dulaglutide - UNII:WTT295HSY5) Dulaglutide 0.75 mg in 0.5 mL Inactive Ingredients Ingredient Name Strength Trisodium Citrate Dihydrate (UNII: B22547B95K) 1.37 mg in 0.5 mL Anhydrous Citric Acid (UNII: XF417D3PSL) 0.07 mg in 0.5 mL Mannitol (UNII: 3OWL53L36A) 23.2 mg in 0.5 mL Polysorbate 80 (UNII: 6OZP39ZG8H) 0.10 mg in 0.5 mL Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0002-1433-80 4 in 1 CARTON 11/07/2014 1 NDC: 0002-1433-01 0.5 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC: 0002-1433-61 2 in 1 CARTON 11/07/2014 2 0.5 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125469 09/18/2014 TRULICITY
dulaglutide injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0002-1434 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Dulaglutide (UNII: WTT295HSY5) (Dulaglutide - UNII:WTT295HSY5) Dulaglutide 1.5 mg in 0.5 mL Inactive Ingredients Ingredient Name Strength Trisodium Citrate Dihydrate (UNII: B22547B95K) 1.37 mg in 0.5 mL Anhydrous Citric Acid (UNII: XF417D3PSL) 0.07 mg in 0.5 mL Mannitol (UNII: 3OWL53L36A) 23.2 mg in 0.5 mL Polysorbate 80 (UNII: 6OZP39ZG8H) 0.10 mg in 0.5 mL Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0002-1434-80 4 in 1 CARTON 11/07/2014 1 NDC: 0002-1434-01 0.5 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC: 0002-1434-61 2 in 1 CARTON 11/07/2014 2 0.5 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125469 09/18/2014 Labeler - Eli Lilly and Company (006421325)
Trademark Results [Trulicity]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TRULICITY 86315558 not registered Dead/Abandoned |
Eli Lilly and Company 2014-06-20 |
 TRULICITY 86314558 not registered Dead/Abandoned |
Eli Lilly and Company 2014-06-19 |
 TRULICITY 86271432 5069090 Live/Registered |
Eli Lilly and Company 2014-05-05 |
 TRULICITY 85183667 4786025 Live/Registered |
Eli Lilly and Company 2010-11-23 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
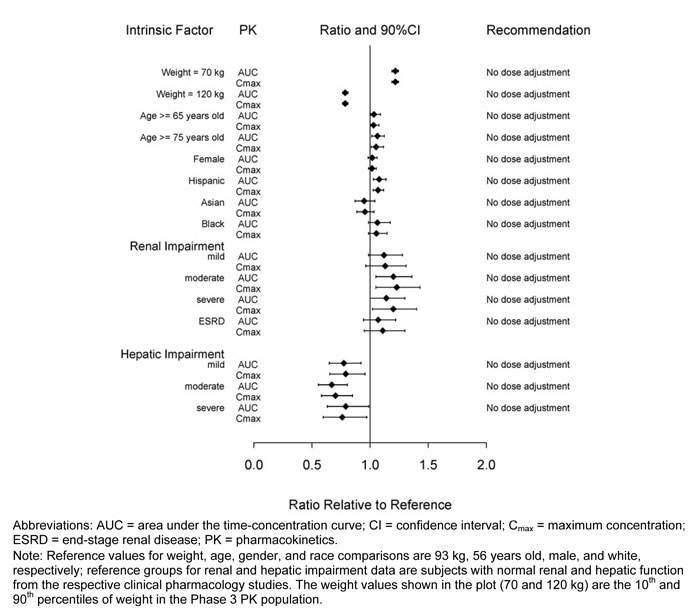
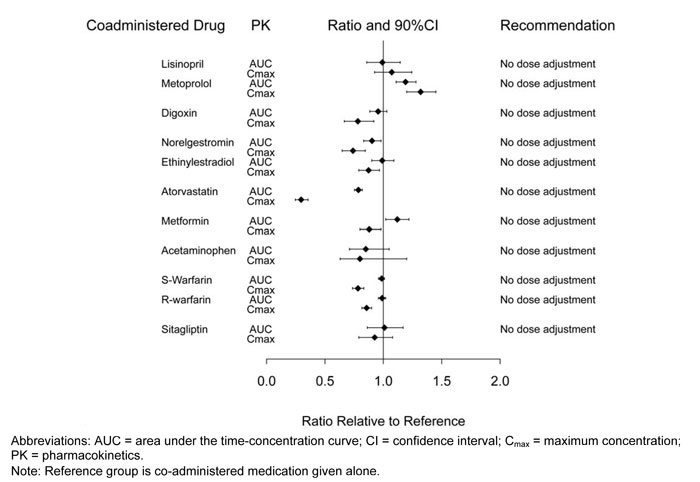
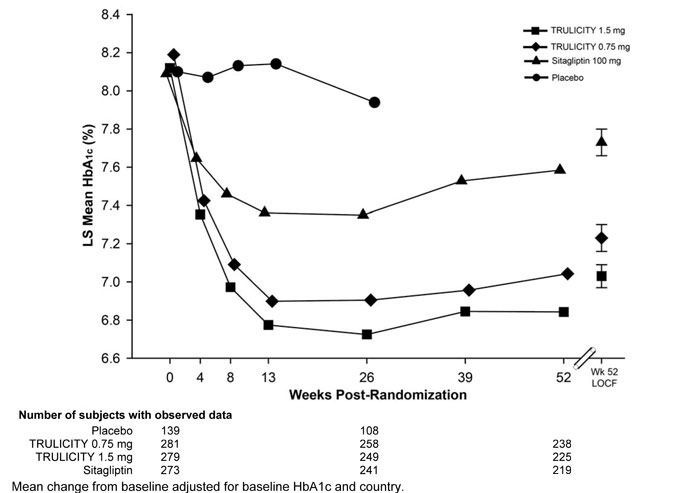
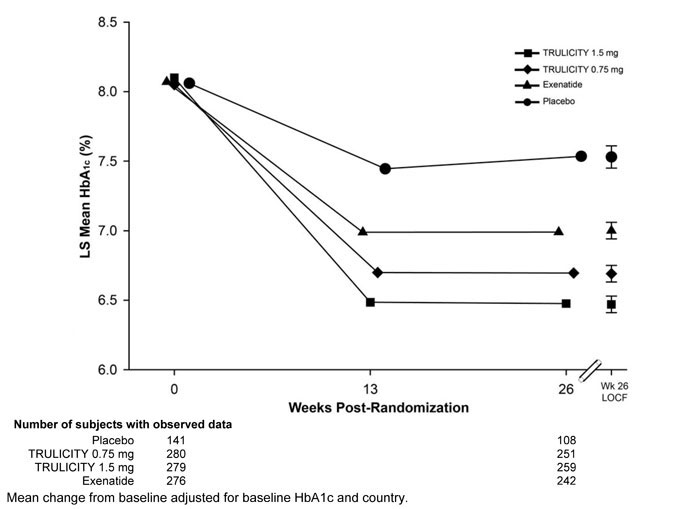
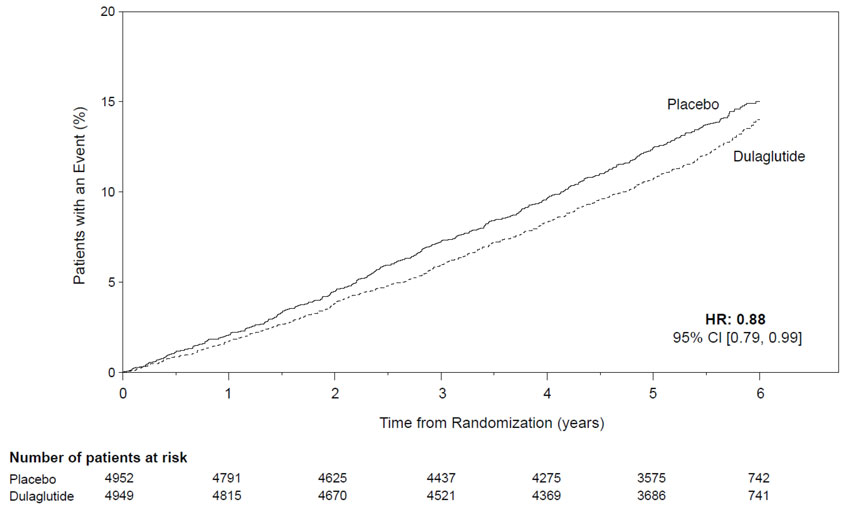







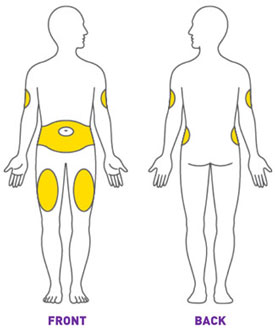
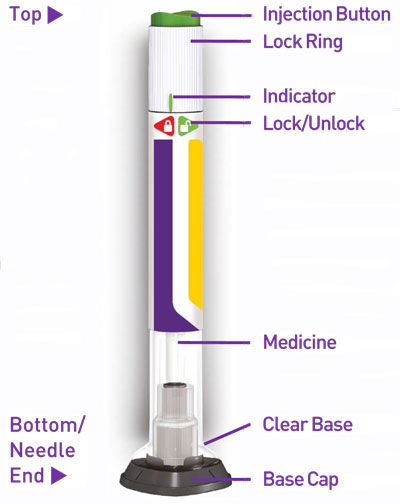
 Make sure the Pen is
Make sure the Pen is 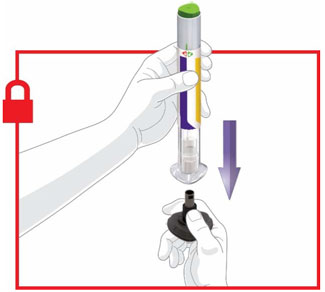

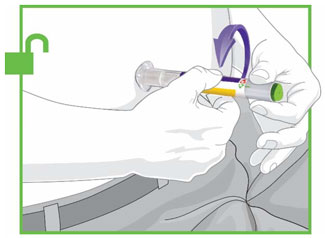
 Continue holding the Clear Base firmly against your skin until you hear a second click. This happens when the needle starts retracting in about 5-10 seconds.
Continue holding the Clear Base firmly against your skin until you hear a second click. This happens when the needle starts retracting in about 5-10 seconds.