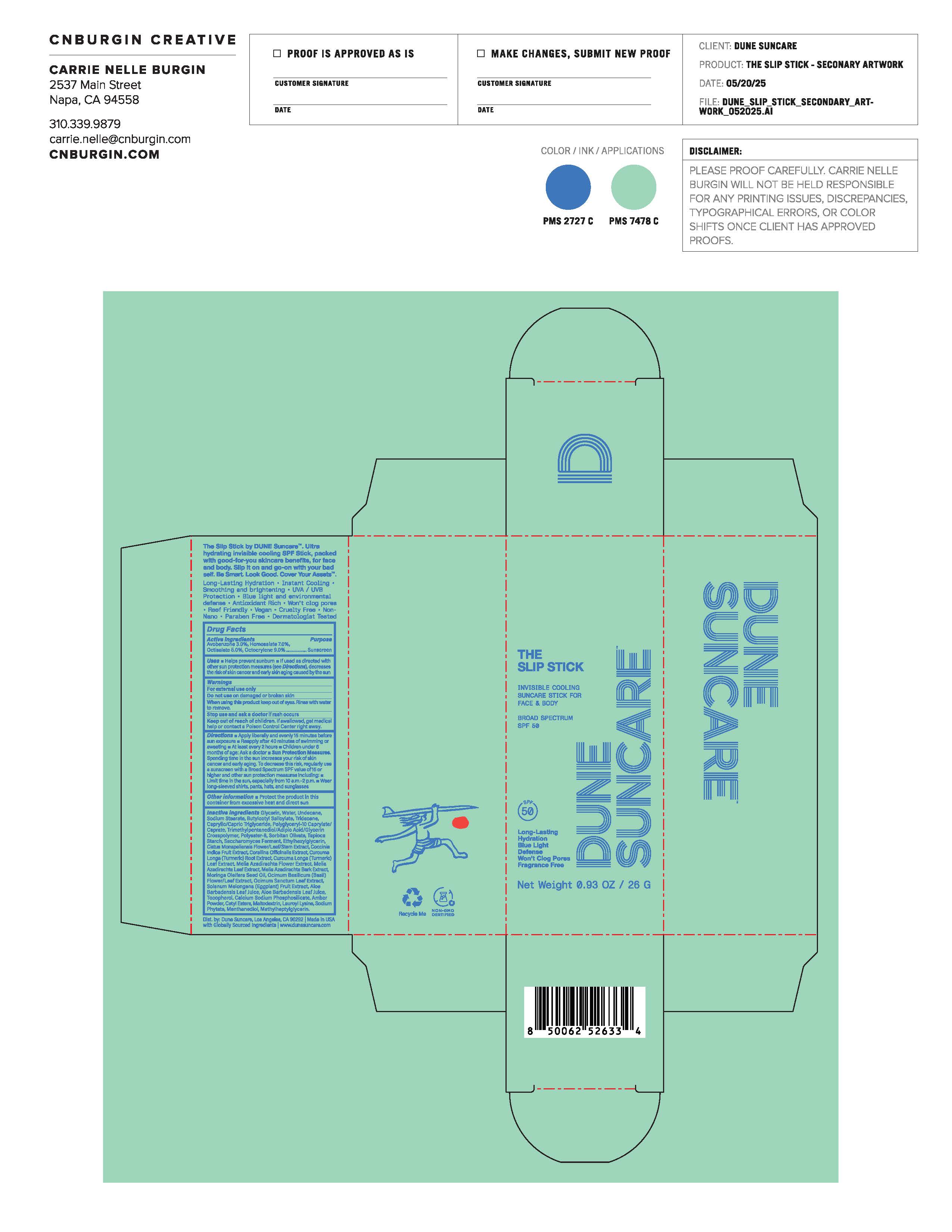
THE SLIP INVISIBLE COOLING SUNCARE FOR FACE AND BODY- avobenzone, homosalate, octisalate, and octocrylene stick
THE SLIP by
Drug Labeling and Warnings
THE SLIP by is a Otc medication manufactured, distributed, or labeled by Dune Suncare, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredients
- Purpose
- Uses
- Warnings
-
Directions
- Apply liberally and evenly 15 minutes before sun exposure
- Reapply after 40 minutes of swimming or sweating
- At least every 2 hours
- Children under 6 months: Ask a doctor
- Sun Protection Measures.Spending time in the sun increases your risk of skin cancer and early aging. To decrease this risk, regularly use a sunscreen with Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m.–2 p.m.
- Wear long-sleeve shirts, pants, hats, and sunglasses
- Other information
-
Inactive ingredients
Glycerin, Water, Undecane, Sodium Stearate, Butyloctyl Salicylate, Tridecane, Caprylic/Capric Triglyceride, Polyglyceryl-10 Caprylate/Caprate, Trimethylpentanediol/Adipic Acid/Glycerin Crosspolymer, Polyester-8, Sorbitan Olivate, Tapioca Starch, Saccharomyces Ferment, Ethylhexylglycerin, Cistus Monspeliensis Flower/Leaf/Stem Extract, Coccinia Indica Fruit Extract, Corallina Officinalis Extract, Curcuma Longa (Turmeric) Root Extract, Curcuma Longa (Turmeric) Leaf Extract, Melia Azadirachta Flower Extract, Melia Azadirachta Leaf Extract, Melia Azadirachta Bark Extract, Moringa Oleifera Seed Oil, Ocimum Basilicum (Basil) Flower/Leaf Extract, Ocimum Sanctum Leaf Extract, Solanum Melongena (Eggplant) Fruit Extract, Aloe Barbadensis Leaf Juice, Aloe Barbadensis Leaf Juice, Tocopherol, Calcium Sodium Phosphosilicate, Amber Powder, Cetyl Esters, Maltodextrin, Lauroyl Lysine, Sodium Phytate, Menthaneidiol, Methylheptylglycerin.
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
THE SLIP INVISIBLE COOLING SUNCARE FOR FACE AND BODY
avobenzone, homosalate, octisalate, and octocrylene stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 82757-206 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 g HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 7 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 9 g in 100 g Inactive Ingredients Ingredient Name Strength CORALLINA OFFICINALIS (UNII: 4004498D06) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) MELIA AZADIRACHTA LEAF (UNII: HKY915780T) TRIMETHYLPENTANEDIOL/ADIPIC ACID/GLYCERIN CROSSPOLYMER (25000 MPA.S) (UNII: 587WKM3S9Q) COCCINIA GRANDIS FRUIT (UNII: VLJ6WOT3K5) CAPRYLIC/CAPRIC TRIGLYCERIDE (UNII: C9H2L21V7U) POLYESTER-8 (1400 MW, CYANODIPHENYLPROPENOYL CAPPED) (UNII: T9296U138P) CURCUMA LONGA LEAF (UNII: H2HC4RY52C) CALCIUM SODIUM PHOSPHOSILICATE (UNII: BXU51RZJ8P) CURCUMA LONGA (TURMERIC) ROOT (UNII: 856YO1Z64F) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) CETYL ESTERS (UNII: D072FFP9GU) LAUROYL LYSINE (UNII: 113171Q70B) SODIUM PHYTATE (UNII: 88496G1ERL) SOLANUM MELONGENA WHOLE (UNII: 5DS5EE0N93) SORBITAN OLIVATE (UNII: MDL271E3GR) SACCHAROMYCES CEREVISIAE (UNII: 978D8U419H) POLYGLYCERYL-10 CAPRYLATE (UNII: YS396CQX5C) AMBER POWDER (UNII: 70J9Z0J26P) MELIA AZEDARACH BARK (UNII: Q38EJM724X) OCIMUM AFRICANUM LEAF (UNII: 0231102WJO) ALOE BARBADENSIS LEAF JUICE (UNII: RUE8E6T4NB) MALTODEXTRIN (UNII: 7CVR7L4A2D) GLYCERIN (UNII: PDC6A3C0OX) METHYLENE GLYCOL (UNII: 6Z20YM9257) UNDECANE (UNII: JV0QT00NUE) WATER (UNII: 059QF0KO0R) SODIUM STEARATE (UNII: QU7E2XA9TG) TRIDECANE (UNII: A3LZF0L939) TAPIOCA STARCH (UNII: 24SC3U704I) MORINGA OLEIFERA SEED OIL (UNII: REM6A5QMC0) OCIMUM BASILICUM FLOWERING TOP (UNII: 7SAB275FP2) CISTUS MONSPELIENSIS WHOLE (UNII: T2OG06826L) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 82757-206-01 1 in 1 CARTON 12/01/2025 1 NDC: 82757-206-00 26 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/01/2025 Labeler - Dune Suncare, Inc. (049095642)
Trademark Results [THE SLIP]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 THE SLIP 85338234 4087413 Dead/Cancelled |
Hobson, Donald 2011-06-04 |
 THE SLIP 75552815 2272338 Dead/Cancelled |
Slip Partnership, The 1998-09-14 |
 THE SLIP 74022825 1681472 Dead/Cancelled |
The Slip 1990-01-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.