GRALISE- gabapentin tablet, film coated GRALISE- gabapentin kit
Gralise by
Drug Labeling and Warnings
Gralise by is a Prescription medication manufactured, distributed, or labeled by Almatica Pharma LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use GRALISE safely and effectively. See full prescribing information for GRALISE.
GRALISE® (gabapentin) tablets, for oral use
Initial U.S. Approval: 1993INDICATIONS AND USAGE
GRALISE is indicated for the management of Postherpetic Neuralgia (PHN).
Important Limitation: GRALISE is not substitutable with other gabapentin products because of differing pharmacokinetic profiles that affect the frequency of administration (See Warnings and Precautions)
DOSAGE AND ADMINISTRATION
- GRALISE should be titrated to an 1,800 mg dose taken orally, once-daily, with the evening meal. GRALISE tablets should be swallowed whole. Do not crush, split, or chew the tablets. (2.1)
- If GRALISE dose is reduced, discontinued, or substituted with an alternative medication, this should be done gradually over a minimum of 1 week or longer (at the discretion of the prescriber). (2.1)
- Renal impairment: Dose should be adjusted in patients with reduced renal function. GRALISE should not be used in patients with CrCl less than 30 or in patients on hemodialysis. (2.2)
DOSAGE FORMS AND STRENGTHS
- Tablets: 300 mg, 450 mg, 600 mg, 750 mg, and 900 mg (3)
CONTRAINDICATIONS
GRALISE is contraindicated in patients who have demonstrated hypersensitivity to the drug or its ingredients. (4)
WARNINGS AND PRECAUTIONS
- GRALISE is not substitutable with other gabapentin products
- Antiepileptic drugs, including gabapentin, the active ingredient in GRALISE, increase the risk of suicidal thoughts or behavior (5.1)
- Abrupt or rapid discontinuation may increase the risk for seizures. Withdrawal symptoms or suicidal behavior and ideation have been observed after discontinuation. Taper GRALISE gradually over a minimum of 1 week. (5.2)
- Respiratory depression may occur with GRALISE when used with concomitant CNS depressants or in the setting of underlying respiratory impairment. Monitor patients and adjust dosage as appropriate. (5.3)
ADVERSE REACTIONS
- The most common adverse reaction (greater than or equal to 5% and twice placebo) is dizziness. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Almatica Pharma LLC at 1-877-447-7979 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
- An increase in gabapentin AUC values have been reported when administered with hydrocodone. (7.6)
- An increase in gabapentin AUC values have been reported when administered with morphine. (7.7)
- An antacid containing aluminum hydroxide and magnesium hydroxide reduced the bioavailability of gabapentin immediate release by about approximately 20%, but by only 5% when gabapentin was taken 2 hours after antacids. It is recommended that GRALISE be taken at least 2 hours following antacid administration. (7.10)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 5/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Postherpetic Neuralgia
2.2 Patients with Renal Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Suicidal Behavior and Ideation
5.2 Increased Risk of Adverse Reactions with Abrupt or Rapid Discontinuation
5.3 Respiratory Depression
5.4 Tumorigenic Potential
5.5 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity
5.6 Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing and Other Experience with other Formulations of Gabapentin
7 DRUG INTERACTIONS
7.1 Phenytoin
7.2 Carbamazepine
7.3 Valproic Acid
7.4 Phenobarbital
7.5 Naproxen
7.6 Hydrocodone
7.7 Morphine
7.8 Cimetidine
7.9 Oral Contraceptives
7.10 Antacid (containing aluminum hydroxide and magnesium hydroxide)
7.11 Probenecid
7.12 Drug/Laboratory Test Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Special Populations
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Postherpetic Neuralgia
Do not use GRALISE as a substitute for other gabapentin products.
Titrate GRALISE to an 1,800 mg dose taken orally once daily with the evening meal. GRALISE tablets should be swallowed whole. Do not split, crush, or chew the tablets.
If GRALISE dosing is reduced, discontinued, or substituted with an alternative medication, this should be done gradually over a minimum of one week or longer (at the discretion of the prescriber).
In adults with postherpetic neuralgia, GRALISE therapy should be initiated and titrated as follows:
Table 1: GRALISE Recommended Titration Schedule Day 1 Day 2 Days 3-6 Days 7-10 Days 11-14 Day 15 Daily Dose 300 mg 600 mg 900 mg 1,200 mg 1,500 mg 1,800 mg 2.2 Patients with Renal Impairment
In patients with stable renal function, creatinine clearance (CCr) can be reasonably well estimated using the equation of Cockcroft and Gault:
For females CCr=(0.85)(140-age)(weight)/[(72)(SCr)]
For males CCr=(140-age)(weight)/[(72)(SCr)]
where age is in years, weight is in kilograms and SCr is serum creatinine in mg/dL.The dose of GRALISE should be adjusted in patients with reduced renal function, according to Table 2. Patients with reduced renal function must initiate GRALISE at a daily dose of 300 mg. GRALISE should be titrated following the schedule outlined in Table 1. Daily dosing in patients with reduced renal function must be individualized based on tolerability and desired clinical benefit.
Table 2: GRALISE Dosage Based on Renal Function Once-daily dosing Creatinine Clearance (mL/min) GRALISE Dose (once daily with evening meal) ≥ 60 1,800 mg 30 - 60 600 mg to 1,800 mg < 30 GRALISE should not be administered patients receiving hemodialysis GRALISE should not be administered -
3 DOSAGE FORMS AND STRENGTHS
Tablets:
- 300 mg, white, oval, debossed with “SLV” on one side and “300” on the other side.
- 450 mg, red, oval, debossed with “ALM” on one side and “450” on the other side.
- 600 mg, beige, oval, debossed with “SLV” on one side and “600” on the other side.
- 750 mg, yellow, oval, debossed with “ALM” on one side and “750” on the other side.
- 900 mg, pink, oval, debossed with “ALM” on one side and “900” on the other side.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
GRALISE is not substitutable with other gabapentin products because of differing pharmacokinetic profiles that affect the frequency of administration.
The safety and effectiveness of GRALISE in patients with epilepsy has not been studied.
5.1 Suicidal Behavior and Ideation
Antiepileptic drugs (AEDs), including gabapentin, the active ingredient in GRALISE, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Suicidal behavior and ideation have also been reported in patients after discontinuation of gabapentin [see Warnings and Precautions (5.3)]. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5-100 years) in the clinical trials analyzed. Table 3 shows absolute and relative risk by indication for all evaluated AEDs.
Table 3: Risk by Indication for Antiepileptic Drugs (including gabapentin, the active ingredient in GRALISE) in the Pooled Analysis Indication Placebo Patients with Events Per 1,000 Patients Drug Patients with Events Per 1,000 Patients Relative Risk: Incidence of Events in Drug Patients/Incidence in Placebo Patients Risk Difference: Additional Drug Patients with Events Per 1,000 Patients Epilepsy 1.0 3.4 3.5 2.4 Psychiatric 5.7 8.5 1.5 2.9 Other 1.0 1.8 1.9 0.9 Total 2.4 4.3 1.8 1.9 The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
Anyone considering prescribing GRALISE must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which products containing active components that are AEDs (such as gabapentin, the active component in GRALISE) are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
Patients, their caregivers, and families should be informed that GRALISE contains gabapentin which is also used to treat epilepsy and that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.
5.2 Increased Risk of Adverse Reactions with Abrupt or Rapid Discontinuation
After discontinuation of short-term and long-term treatment with gabapentin, withdrawal symptoms have been observed in some patients [see Adverse Reactions (6.2) and Drug Abuse and Dependence (9.3)]. Suicidal behavior and ideation have also been reported in patients after discontinuation of gabapentin [see Warnings and Precautions (5.1)]. If GRALISE is discontinued, this should be done gradually over a minimum of 1 week or longer (at the discretion of the prescriber).
5.3 Respiratory Depression
There is evidence from case reports, human studies, and animal studies associating gabapentin with serious, life-threatening, or fatal respiratory depression when co-administrated with central nervous system (CNS) depressants, including opioids, or in the setting of underlying respiratory impairment. When the decision is made to co-prescribe GRALISE with another CNS depressant, particularly an opioid, or to prescribe GRALISE to patients with underlying respiratory impairment, monitor patients for symptoms of respiratory depression and sedation, and consider initiating GRALISE at a low dose. The management of respiratory depression may include close observation, supportive measures, and reduction or withdrawal of CNS depressants (including GRALISE).
5.4 Tumorigenic Potential
In standard preclinical in vivo lifetime carcinogenicity studies, an unexpectedly high incidence of pancreatic acinar adenocarcinomas was identified in male, but not female, rats. The clinical significance of this finding is unknown.
In clinical trials of gabapentin therapy in epilepsy comprising 2,085 patient-years of exposure in patients over 12 years of age, new tumors were reported in 10 patients, and pre-existing tumors worsened in 11 patients, during or within 2 years after discontinuing the drug. However, no similar patient population untreated with gabapentin was available to provide background tumor incidence and recurrence information for comparison. Therefore, the effect of gabapentin therapy on the incidence of new tumors in humans or on the worsening or recurrence of previously diagnosed tumors is unknown.
5.5 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as Multiorgan Hypersensitivity, has been reported in patients taking antiepileptic drugs, including GRALISE. Some of these events have been fatal or life-threatening. DRESS typically, although not exclusively, presents with fever, rash, and/or lymphadenopathy in association with other organ system involvement, such as hepatitis, nephritis, hematological abnormalities, myocarditis, or myositis sometimes resembling an acute viral infection. Eosinophilia is often present. Because this disorder is variable in its expression, other organ systems not noted here may be involved.
It is important to note that early manifestations of hypersensitivity, such as fever or lymphadenopathy, may be present even though rash is not evident. If such signs or symptoms are present, the patient should be evaluated immediately. GRALISE should be discontinued if an alternative etiology for the signs or symptoms cannot be established.
-
6 ADVERSE REACTIONS
The following adverse reactions are described elsewhere in the labeling:
- Suicidal Behavior and Ideation [see Warnings and Precautions (5.1)]
- Increased Risk of Adverse Reactions with Abrupt or Rapid Discontinuation [see Warnings and Precautions (5.2)]
- Respiratory Depression [see Warnings and Precautions (5.3)]
- Tumorigenic Potential [see Warnings and Precautions (5.4)]
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity [see Warnings and Precautions (5.5)]
- Laboratory Tests [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
A total of 359 patients with neuropathic pain associated with postherpetic neuralgia have received GRALISE at doses up to 1,800 mg daily during placebo-controlled clinical studies. In clinical trials in patients with postherpetic neuralgia, 9.7% of the 359 patients treated with GRALISE and 6.9% of 364 patients treated with placebo discontinued prematurely due to adverse reactions. In the GRALISE treatment group, the most common reason for discontinuation due to adverse reactions was dizziness. Of GRALISE-treated patients who experienced adverse reactions in clinical studies, the majority of those adverse reactions were either "mild" or "moderate".
Table 4 lists all adverse reactions, regardless of causality, occurring in at least 1% of patients with neuropathic pain associated with postherpetic neuralgia in the GRALISE group for which the incidence was greater than in the placebo group.
Table 4: Treatment-Emergent Adverse Reaction Incidence in Controlled Trials in Neuropathic Pain Associated with Postherpetic Neuralgia (Events in at Least 1% of all GRALISE-Treated Patients and More Frequent Than in the Placebo Group) Body System - Preferred Term GRALISE
N = 359
%Placebo
N = 364
%Ear and Labyrinth Disorders Vertigo 1.4 0.5 Gastrointestinal Disorders Diarrhea 3.3 2.7 Dry mouth 2.8 1.4 Constipation 1.4 0.3 Dyspepsia 1.4 0.8 General Disorders Peripheral edema 3.9 0.3 Pain 1.1 0.5 Infections and Infestations Nasopharyngitis 2.5 2.2 Urinary tract infection 1.7 0.5 Investigations Weight increased 1.9 0.5 Musculoskeletal and Connective Tissue Disorders Pain in extremity 1.9 0.5 Back pain 1.7 1.1 Nervous System Disorders Dizziness 10.9 2.2 Somnolence 4.5 2.7 Headache 4.2 4.1 Lethargy 1.1 0.3 In addition to the adverse reactions reported in Table 4 above, the following adverse reactions with an uncertain relationship to GRALISE were reported during the clinical development for the treatment of postherpetic neuralgia. Events in more than 1% of patients but equally or more frequently in the GRALISE-treated patients than in the placebo group included blood pressure increase, confusional state, gastroenteritis viral, herpes zoster, hypertension, joint swelling, memory impairment, nausea, pneumonia, pyrexia, rash, seasonal allergy, and upper respiratory infection.
6.2 Postmarketing and Other Experience with other Formulations of Gabapentin
In addition to the adverse experiences reported during clinical testing of gabapentin, the following adverse experiences have been reported in patients receiving other formulations of marketed gabapentin. These adverse experiences have not been listed above and data are insufficient to support an estimate of their incidence or to establish causation. The listing is alphabetized: angioedema, blood glucose fluctuation, breast enlargement, bullous pemphigoid, elevated creatine kinase, elevated liver function tests, erythema multiforme, fever, hyponatremia, jaundice, movement disorder, Stevens-Johnson syndrome.
There are postmarketing reports of withdrawal symptoms after discontinuation of gabapentin. Reported adverse reactions include, but are not limited to, seizures, depression, suicidal ideationand behavior, agitation, confusion, disorientation, psychotic symptoms, anxiety, insomnia, nausea, pain, sweating, tremor, headache, dizziness, and malaise [see Warnings and Precautions(5.2)].
There are postmarketing reports of life-threatening or fatal respiratory depression in patients taking gabapentin with opioids or other central nervous system (CNS) depressants, or in the setting of underlying respiratory impairment.
-
7 DRUG INTERACTIONS
In vitro studies were conducted to investigate the potential of gabapentin to inhibit the major cytochrome P450 enzymes (CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4) that mediate drug and xenobiotic metabolism using isoform selective marker substrates and human liver microsomal preparations. Only at the highest concentration tested (171 mcg/mL; 1mM) was a slight degree of inhibition (14% to 30%) of isoform CYP2A6 observed. No inhibition of any of the other isoforms tested was observed at gabapentin concentrations up to 171 mcg/mL (approximately 15 times the Cmax at 3,600 mg/day).
Gabapentin is not appreciably metabolized nor does it interfere with the metabolism of commonly coadministered antiepileptic drugs.
The drug interaction data described in this section were obtained from studies involving healthy adults and adult patients with epilepsy.
7.1 Phenytoin
In a single (400 mg) and multiple dose (400 mg three times daily) study of gabapentin immediate release in epileptic patients (N=8) maintained on phenytoin monotherapy for at least 2 months, gabapentin had no effect on the steady-state trough plasma concentrations of phenytoin and phenytoin had no effect on gabapentin pharmacokinetics.
7.2 Carbamazepine
Steady-state trough plasma carbamazepine and carbamazepine 10, 11 epoxide concentrations were not affected by concomitant gabapentin immediate release (400 mg three times daily; N=12) administration. Likewise, gabapentin pharmacokinetics were unaltered by carbamazepine administration.
7.3 Valproic Acid
The mean steady-state trough serum valproic acid concentrations prior to and during concomitant gabapentin immediate release administration (400 mg three times daily; N=17) were not different and neither were gabapentin pharmacokinetic parameters affected by valproic acid.
7.4 Phenobarbital
Estimates of steady-state pharmacokinetic parameters for phenobarbital or gabapentin immediate release (300 mg three times daily; N=12) are identical whether the drugs are administered alone or together.
7.5 Naproxen
Coadministration of single doses of naproxen (250 mg) and gabapentin immediate release (125 mg) to 18 volunteers increased gabapentin absorption by 12% to 15%. Gabapentin immediate release had no effect on naproxen pharmacokinetics. The doses are lower than the therapeutic doses for both drugs. The effect of coadministration of these drugs at therapeutic doses is not known.
7.6 Hydrocodone
Coadministration of gabapentin immediate release (125 mg and 500 mg) and hydrocodone (10 mg) reduced hydrocodone Cmax by 3% and 21%, respectively, and AUC by 4% and 22%, respectively. The mechanism of this interaction is unknown. Gabapentin AUC values were increased by 14%; the magnitude of the interaction at other doses is not known.
7.7 Morphine
When a single dose (60 mg) of controlled-release morphine capsule was administered 2 hours prior to a single dose (600 mg) of gabapentin immediate release in 12 volunteers, mean gabapentin AUC values increased by 44% compared to gabapentin immediate release administered without morphine. The pharmacokinetics of morphine were not affected by administration of gabapentin immediate release 2 hours after morphine. The magnitude of this interaction at other doses is not known.
7.8 Cimetidine
Cimetidine 300 mg decreased the apparent oral clearance of gabapentin by 14% and creatinine clearance by 10%. The effect of gabapentin immediate release on cimetidine was not evaluated. This decrease is not expected to be clinically significant.
7.9 Oral Contraceptives
Gabapentin immediate release (400 mg three times daily) had no effect on the pharmacokinetics of norethindrone (2.5 mg) or ethinyl estradiol (50 mcg) administered as a single tablet, except that the Cmax of norethindrone was increased by 13%. This interaction is not considered to be clinically significant.
7.10 Antacid (containing aluminum hydroxide and magnesium hydroxide)
An antacid containing aluminum hydroxide and magnesium hydroxide reduced the bioavailability of gabapentin immediate release by about approximately 20%, but by only 5% when gabapentin immediate release was taken 2 hours after the antacid. It is recommended that GRALISE be taken at least 2 hours following the antacid (containing aluminum hydroxide and magnesium hydroxide) administration.
7.11 Probenecid
Gabapentin immediate release pharmacokinetic parameters were comparable with and without probenecid, indicating that gabapentin does not undergo renal tubular secretion by the pathway that is blocked by probenecid.
7.12 Drug/Laboratory Test Interactions
False positive readings were reported with the Ames-N-Multistix SG® dipstick test for urine protein when gabapentin was added to other antiepileptic drugs; therefore, the more specific sulfosalicylic acid precipitation procedure is recommended to determine the presence of urine protein.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to gabapentin, including GRALISE, during pregnancy. Encourage women who are taking GRALISE during pregnancy to enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry by calling the toll free number 1-888-233-2334 or visiting https://www.aedpregnancyregistry.org/.
Risk Summary
Available data from published prospective and retrospective cohort studies, and case reports over decades of use with gabapentin during pregnancy have not identified a drug-associated risk of major birth defects. The available data are insufficient to evaluate a drug-associated risk of miscarriage and other maternal or fetal outcomes. In nonclinical studies in mice, rats, and rabbits, gabapentin was developmentally toxic (increased fetal skeletal and visceral abnormalities, and increased embryofetal mortality) when administered to pregnant animals at doses similar to those used clinically (see Data).
Postmarketing data suggest that extended gabapentin use with opioids close to delivery mayincrease the risk of neonatal withdrawal versus opioids alone [see Clinical Considerations]. Although there is at least one report of neonatal withdrawal syndrome in an infant exposed togabapentin alone during pregnancy, there are no comparative epidemiologic studies evaluatingthis association. Therefore, it is not known whether exposure to gabapentin alone late inpregnancy may cause withdrawal signs and symptoms.
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Neonatal withdrawal syndrome has been reported in newborns exposed to gabapentin in utero foran extended period of time when also exposed to opioids close to delivery. Neonatal withdrawal signs and symptoms reported have included tachypnea, vomiting, diarrhea, hypertonia, irritability, sneezing, poor feeding, hyperactivity, abnormal sleep pattern, and tremor. Reported signs and symptoms that may also be related to withdrawal include tongue thrusting, wandering eye movements while awake, back arching, and continuous extremity movements. Observe neonates exposed to GRALISE and opioids for signs and symptoms of neonatal withdrawal and manage accordingly.
Data
Animal Data
When pregnant mice received oral doses of gabapentin (1,000 or 3,000 mg/kg/day, approximately 3 to 8 times the maximum recommended dose of 1,800 mg on a mg/m2 basis) during the period of organogenesis, embryofetal toxicity (increased incidences of skeletal variations) was observed. The no effect level was 500 mg/kg/day, representing approximately the maximum recommended human dose [MRHD] on a mg/m2 basis.
When rats were dosed prior to and during mating, and throughout gestation, pups from all dose groups (500, 1,000 and 2,000 mg/kg/day) were affected. These doses are equivalent to approximately 3 to 11 times the MRHD on a mg/m2 basis. There was an increased incidence of hydroureter and/or hydronephrosis in rats in a study of fertility and general reproductive performance at 2,000 mg/kg/day with no effect at 1,000 mg/kg/day, in a teratology study at 1,500 mg/kg/day with no effect at 300 mg/kg/day, and in a perinatal and postnatal study at all doses studied (500, 1,000 and 2,000 mg/kg/day). The doses at which the effects occurred are approximately 3 to 11 times the maximum recommended dose of 1,800 mg on a mg/m2 basis; the no-effect doses were approximately 5 times (Fertility and General Reproductive Performance study) and approximately equal to (Teratogenicity study) the MRHD on a mg/m2 basis. Other than hydroureter and hydronephrosis, the etiologies of which are unclear, the incidence of malformations was not increased compared to controls in offspring of mice, rats, or rabbits given doses up to 8 times (mice), 10 times (rats), or 16 times (rabbits) the human daily dose on a mg/m2 basis.
When pregnant rabbits were treated with gabapentin during the period of organogenesis, an increase in embryofetal mortality was observed at 60, 300, and 1,500 mg/kg/day (0.6 to 16 times the MRHD on a mg/m2 basis).
In a published study, gabapentin (400 mg/kg/day) was administered by intraperitoneal injection to neonatal mice during the first postnatal week, a period of synaptogenesis in rodents (corresponding to the last trimester of pregnancy in humans). Gabapentin caused a marked decrease in neuronal synapse formation in brains of intact mice and abnormal neuronal synapse formation in a mouse model of synaptic repair. Gabapentin has been shown in vitro to interfere with activity of the α2δ subunit of voltage-activated calcium channels, a receptor involved in neuronal synaptogenesis. The clinical significance of these findings is unknown.
8.2 Lactation
Risk Summary
Gabapentin is present in human milk following oral administration. Adverse effects on the breastfed infant have not been reported. There are no data on the effects of the drug on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for GRALISE and any potential adverse effects on the breastfed infant from GRALISE or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of GRALISE in the management of postherpetic neuralgia in patients less than 18 years of age has not been studied.
8.5 Geriatric Use
The total number of patients treated with GRALISE in controlled clinical trials in patients with postherpetic neuralgia was 359, of which 63% were 65 years of age or older. The types and incidence of adverse events were similar across age groups except for peripheral edema, which tended to increase in incidence with age.
GRALISE is known to be substantially excreted by the kidney. Reductions in GRALISE dose should be made in patients with age-related compromised renal function [see Dosage and Administration (2.2)].
8.6 Hepatic Impairment
Because gabapentin is not metabolized, studies have not been conducted in patients with hepatic impairment.
8.7 Renal Impairment
GRALISE is known to be substantially excreted by the kidney. Dosage adjustment is necessary in patients with impaired renal function. GRALISE should not be administered in patients with CrCL between 15 and 30 or in patients undergoing hemodialysis [see Dosage and Administration (2.2)].
-
9 DRUG ABUSE AND DEPENDENCE
9.3 Dependence
Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug.
After discontinuation of short-term and long-term treatment with gabapentin, withdrawal symptoms have been observed in some patients. Withdrawal symptoms may occur shortly after discontinuation, usually within 48 hours. In the postmarketing setting, reported adverse reactions have included, but not been limited to, seizures, depression, suicidal ideation and behavior,agitation, confusion, disorientation, psychotic symptoms, anxiety, insomnia, nausea, pain,sweating, tremor, headache, dizziness, and malaise. The abuse and dependence potential of GRALISE has not been evaluated in human studies.
-
10 OVERDOSAGE
Signs of acute toxicity in animals included ataxia, labored breathing, ptosis, sedation, hypoactivity, or excitation.
Acute oral overdoses of gabapentin have been reported. Symptoms include double-vision, tremor, slurred speech, drowsiness, altered mental status, dizziness, lethargy, and diarrhea. Fatal respiratory depression has been reported with gabapentin overdose, alone and in combination with other central nervous system (CNS) depressants.
Gabapentin can be removed by hemodialysis. Hemodialysis has been performed in overdose cases reported, and it may be indicated by the patient’s clinical state or in patients with significant renal impairment.
-
11 DESCRIPTION
GRALISE contains gabapentin, a gamma-aminobutyric acid (GABA) analogue, as the active pharmaceutical ingredient. Gabapentin's chemical name is 1-(aminomethyl)cyclohexaneacetic acid; with a molecular formula of C9H17NO2 and a molecular weight of 171.24 g/mol.
Gabapentin chemical structural formula is:
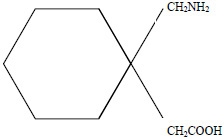
Gabapentin is a white to off-white crystalline solid with a pKa1 of 3.7 and a pKa2 of 10.7. It is freely soluble in water and acidic and basic solutions. The log of the partition coefficient (n-octanol/ 0.05M phosphate buffer) at pH 7.4 is -1.25.
GRALISE is intended for oral administration and is supplied as tablets containing 300 mg, 450 mg, 600 mg, 750 mg, or 900 mg of gabapentin USP.
Each 300 mg tablet contains the inactive ingredients Copovidone NF, Hypromellose USP, Magnesium Stearate NF, Microcrystalline Cellulose NF, Polyethylene Oxide NF, and white film coating (Polyvinyl Alcohol USP, Talc USP, Titanium Oxide USP, Polyethylene Glycol 3350 USP, and Lecithin NF (soya)).
Each 450 mg tablet contains the inactive ingredients Copovidone NF, Hypromellose USP, Magnesium Stearate NF, Polyethylene Oxide NF, and red film coating (Polyethylene glycol 3350 USP, Polyvinyl Alcohol USP, Talc USP, Titanium Oxide USP, and Iron Oxide Red NF).
Each 600 mg tablet contains the inactive ingredients Copovidone NF, Hypromellose USP, Magnesium Stearate NF, Polyethylene Oxide NF, and beige film coating (Polyethylene Glycol 3350 USP, Polyvinyl Alcohol USP, Talc USP, Titanium Oxide USP, Iron Oxide Yellow NF, and Iron Oxide Red NF).
Each 750 mg tablet contains the inactive ingredients Copovidone NF, Hypromellose USP, Magnesium Stearate NF, Polyethylene Oxide NF, and yellow film coating (Polyethylene Glycol 3350 USP, Polyvinyl Alcohol USP, Talc USP, Titanium oxide USP, and Iron Oxide Yellow NF).
Each 900 mg tablet contains the inactive ingredients Copovidone NF, Hypromellose USP, Magnesium Stearate NF, Polyethylene Oxide NF, and pink film coating (Polyethylene Glycol 3350 USP, Polyvinyl Alcohol USP, Talc USP, Titanium oxide USP, and Iron Oxide Red NF).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action by which gabapentin exerts its analgesic action is unknown but in animal models of analgesia, gabapentin prevents allodynia (pain-related behavior in response to a normally innocuous stimulus) and hyperalgesia (exaggerated response to painful stimuli). Gabapentin prevents pain-related responses in several models of neuropathic pain in rats and mice (e.g., spinal nerve ligation models, spinal cord injury model, acute herpes zoster infection model). Gabapentin also decreases pain-related responses after peripheral inflammation (carrageenan footpad test, late phase of formulin test), but does not alter immediate pain-related behaviors (rat tail flick test, formalin footpad acute phase). The relevance of these models to human pain is not known.
Gabapentin is structurally related to the neurotransmitter GABA (gamma-aminobutyric acid), but it does not modify GABAA or GABAB radioligand binding, it is not converted metabolically into GABA or a GABA agonist, and it is not an inhibitor of GABA uptake or degradation. In radioligand binding assays at concentrations up to 100 μM, gabapentin did not exhibit affinity for a number of other receptor sites, including benzodiazepine, glutamate, N-methyl-D-aspartate (NMDA), quisqualate, kainate, strychnine-insensitive or strychnine-sensitive glycine; alpha 1, alpha 2, or beta adrenergic; adenosine A1 or A2; cholinergic, muscarinic, or nicotinic; dopamine D1 or D2; histamine H1; serotonin S1 or S2; opiate mu, delta, or kappa; cannabinoid 1; voltage-sensitive calcium channel sites labeled with nitrendipine or diltiazem; or at voltage-sensitive sodium channel sites labeled with batrachotoxinin A20-alpha-benzoate. Gabapentin did not alter the cellular uptake of dopamine, noradrenaline, or serotonin.
In vitro studies with radiolabeled gabapentin have revealed a gabapentin binding site in areas of rat brain including neocortex and hippocampus. A high-affinity binding protein in animal brain tissue has been identified as an auxiliary subunit of voltage-activated calcium channels. However, functional correlates of gabapentin binding, if any, remain to be elucidated. It is hypothesized that gabapentin antagonizes thrombospondin binding to α2δ-1 as a receptor involved in excitatory synapse formation and suggested that gabapentin may function therapeutically by blocking new synapse formation.
12.3 Pharmacokinetics
Absorption
Gabapentin is absorbed from the proximal small bowel by a saturable L-amino transport system. Gabapentin bioavailability is not dose proportional; as the dose is increased, bioavailability decreases.
When GRALISE (1,800 mg once daily) and gabapentin immediate release (600 mg three times a day) were administered with high fat meals (50% of calories from fat), GRALISE has a higher Cmax and lower AUC at steady state compared to gabapentin immediate release (Table 5). Time to reach maximum plasma concentration (Tmax) for GRALISE is 8 hours, which is about 4-6 hours longer compared to gabapentin immediate release.
Table 5: Mean ± SD Steady-State Pharmacokinetics for GRALISE and Gabapentin Immediate Release in Healthy Subjects under high-fat high calorie fed state (Day 5, n = 21) Pharmacokinetic
Parameter
(Mean ± SD)GRALISE
1,800 mg QD (3 x 600 mg)Gabapentin Immediate Release
600 mg TID$Tmax is presented as median (range); * relative to most recent dose AUC0-24 (μg.hr/mL) 132.8 ± 34.7 141.3 ± 29.8 Cmax (μg/mL) 9.59 ± 2.33 8.54 ± 1.72 Cmin (μg/mL) 1.84 ± 0.65 2.6 ± 0.78 Tmax (hr)$ 8 (3-12) 2 (1-5)* The single dose pharmacokinetic parameters of 900 mg strength under high fat-high calorie fed state and low fat-low calorie fed state are presented shown in Table 6.
Table 6: Mean ± SD Single-Dose Pharmacokinetic parameters for GRALISE 900 mg strength in Healthy Subjects (n = 27) $Tmax is presented as median (range) Pharmacokinetic Parameter
(Mean ± SD)GRALISE 1,800 mg (2 x 900 mg tablets) High fat- high calorie fed state Low fat- low calorie fed state Cmax (μg/mL) 9.49 ± 1.93 6.50 ± 2.16 AUCt (μg.hr/mL) 127.2 ± 42.8 79.1 ± 39.4 AUCinf (μg.hr/mL) 133.2 ± 43.2 84.8 ± 39.4 Tmax (hr)$ 7 (4-12) 4 (3-12) Do not use GRALISE as a substitute for other gabapentin products because of differing pharmacokinetic profiles that affect frequency of administration.
GRALISE should be taken with evening meals. If it is taken on an empty stomach, the bioavailability will be substantially lower.
Administration of GRALISE with food increases the rate and extent of absorption of gabapentin compared to the fasted state. Cmax of gabapentin increases 33-84% and AUC of gabapentin increases 33-118% with food depending on the fat content of the meal. GRALISE should be taken with food.
Distribution
Gabapentin is less than 3% bound to plasma proteins. After 150 mg intravenous administration, the mean ± SD volume of distribution is 58 ± 6 L.
Elimination
Gabapentin is eliminated by renal excretion as unchanged drug.
In patients with normal renal function given gabapentin immediate release 1,200 to 3,000 mg/day, the drug elimination half-life (t1/2) was 5 to 7 hours. Elimination kinetics do not change with dose level or multiple doses.
Metabolism
Gabapentin is not appreciably metabolized in humans.
Excretion
Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly proportional to creatinine clearance. In elderly patients and patients with impaired renal function, plasma clearance is reduced. Gabapentin can be removed from plasma by hemodialysis.
Dosage adjustment in patients with compromised renal function is necessary. In patients undergoing hemodialysis, GRALISE should not be administered [see Dosage and Administration (2.2)].
12.4 Special Populations
Renal Insufficiency: As renal function decreases, renal and plasma clearances and the apparent elimination rate constant decrease, while Cmax and t1/2 increase.
In patients (N=60) with creatinine clearance of at least 60, 30 to 59, or less than 30 mL/min, the median renal clearance rates for a 400 mg single dose of gabapentin immediate release were 79, 36, and 11 mL/min, respectively, and the median t1/2 values were 9.2, 14, and 40 hours, respectively.
Dosage adjustment is necessary in patients with impaired renal function [see Dosage and Administration (2.2)].
Hemodialysis: In a study in anuric adult subjects (N=11), the apparent elimination half-life of gabapentin on nondialysis days was about 132 hours; during dialysis the apparent half-life of gabapentin was reduced to 3.8 hours. Hemodialysis thus has a significant effect on gabapentin elimination in anuric subjects. GRALISE should not be administered in patients undergoing hemodialysis. Alternative formulations of gabapentin products should be considered in patients undergoing hemodialysis.
Elderly: Apparent oral and renal clearances of gabapentin decrease with increasing age, although this may be related to the decline in renal function with age. Reductions in gabapentin dose should be made in patients with age-related compromised renal function [see Dosage and Administration (2.2)].
Hepatic Impairment: Because gabapentin is not metabolized, studies have not been conducted in patients with hepatic impairment.
Pediatrics: The pharmacokinetics of GRALISE have not been studied in patients less than 18 years of age.
Gender: Although no formal study has been conducted to compare the pharmacokinetics of gabapentin in men and women, it appears that the pharmacokinetic parameters for males and females are similar and there are no significant gender differences.
Race: Pharmacokinetic differences due to race have not been studied. Because gabapentin is primarily renally excreted and there are no important racial differences in creatinine clearance, pharmacokinetic differences due to race are not expected.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Gabapentin was given in the diet to mice at 200, 600, and 2,000 mg/kg/day and to rats at 250, 1,000, and 2,000 mg/kg/day for 2 years. A statistically significant increase in the incidence of pancreatic acinar cell adenoma and carcinomas was found in male rats receiving the high dose; the no-effect dose for the occurrence of carcinomas was 1,000 mg/kg/day. Peak plasma concentrations of gabapentin in rats receiving the high dose of 2,000 mg/kg/day were more than 10 times higher than plasma concentrations in humans receiving 1,800 mg per day and in rats receiving 1,000 mg/kg/day peak plasma concentrations were more than 6.5 times higher than in humans receiving 1,800 mg/day. The pancreatic acinar cell carcinomas did not affect survival, did not metastasize and were not locally invasive. The relevance of this finding to carcinogenic risk in humans is unclear.
Studies designed to investigate the mechanism of gabapentin-induced pancreatic carcinogenesis in rats indicate that gabapentin stimulates DNA synthesis in rat pancreatic acinar cells in vitro and, thus, may be acting as a tumor promoter by enhancing mitogenic activity. It is not known whether gabapentin has the ability to increase cell proliferation in other cell types or in other species, including humans.
Mutagenesis
Gabapentin did not demonstrate mutagenic or genotoxic potential in 3 in vitro and 4 in vivo assays. It was negative in the Ames test and the in vitro HGPRT forward mutation assay in Chinese hamster lung cells; it did not produce significant increases in chromosomal aberrations in the in vitro Chinese hamster lung cell assay; it was negative in the in vivo chromosomal aberration assay and in the in vivo micronucleus test in Chinese hamster bone marrow; it was negative in the in vivo mouse micronucleus assay; and it did not induce unscheduled DNA synthesis in hepatocytes from rats given gabapentin.
Impairment of Fertility
No adverse effects on fertility or reproduction were observed in rats at doses up to 2,000 mg/kg (approximately 11 times the maximum recommended human dose on an mg/m2 basis).
-
14 CLINICAL STUDIES
The efficacy of GRALISE for the management of postherpetic neuralgia was established in a double-blind, placebo-controlled, multicenter study. This study enrolled patients between the age of 21 to 89 with postherpetic neuralgia persisting for at least 6 months following healing of herpes zoster rash and a minimum baseline pain intensity score of at least 4 on an 11-point numerical pain rating scale ranging from 0 (no pain) to 10 (worst possible pain).
This 11-week study compared GRALISE 1,800 mg once daily with placebo. A total of 221 and 231 patients were treated with GRALISE or placebo, respectively. The study treatment including titration for all patients comprised a 10-week treatment period followed by 1-week of dose tapering. Double-blind treatment began with titration starting at 300 mg/day and titrated up to a total daily dose of 1,800 mg over 2 weeks, followed by 8 weeks fixed dosing at 1,800 mg once daily, and then 1 week of dose tapering. During the 8-week stable dosing period, patients took 3 active or placebo tablets each night with the evening meal. During baseline and treatment, patients recorded their pain in a daily diary using an 11-point numeric pain rating scale. The mean baseline pain score was 6.6 and 6.5 for GRALISE and placebo-treated patients, respectively.
Treatment with GRALISE statistically significantly improved the endpoint mean pain score from baseline. For various degrees of improvement in pain from baseline to study endpoint, Figure 1 shows the fraction of patients achieving that degree of improvement. The figure is cumulative, so that patients whose change from baseline is, for example, 50%, are also included at every level of improvement below 50%. Patients who did not complete the study were assigned 0% improvement.
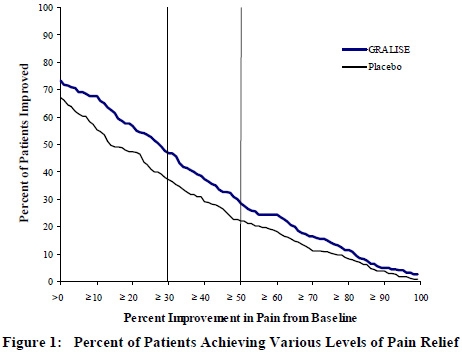
-
16 HOW SUPPLIED/STORAGE AND HANDLING
GRALISE (gabapentin) Tablets are supplied as follows:
300 mg tablets:
GRALISE 300 mg tablets are white, oval, debossed with “SLV” on one side and “300” on the other side.
NDC: 52427-803-90 (Bottle of 90)450 mg tablets:
GRALISE 450 mg tablets are red, oval, debossed with “ALM” on one side and “450” on the other side.
NDC: 52427-804-60 (Bottle of 60)600 mg tablets:
GRALISE 600 mg tablets are beige, oval, debossed with “SLV” on one side and “600” on the other side.
NDC: 52427-806-90 (Bottle of 90)750 mg tablets:
GRALISE 750 mg tablets are yellow, oval, debossed with “ALM” on one side and “750” on the other side.
NDC: 52427-850-60 (Bottle of 60)900 mg tablets:
GRALISE 900 mg tablets are pink, oval, debossed with “ALM” on one side and “900” on
NDC: 52427-890-60 (Bottle of 60)Storage
Store at 20ºC to 25ºC (68ºF to 77ºF); excursions permitted to 15ºC to 30ºC (59ºF to 86ºF) [see USP Controlled Room Temperature].
Keep out of reach of children.
-
17 PATIENT COUNSELING INFORMATION
Advise patients of the availability of a Medication Guide, and instruct them to read the Medication Guide prior to taking GRALISE.
- Advise patients that GRALISE is not substitutable with other formulations of gabapentin.
- Advise patients to take GRALISE only as prescribed. GRALISE may cause dizziness, somnolence, and other signs and symptoms of CNS depression.
- Advise patients not to drive or operate other complex machinery until they have gained sufficient experience on GRALISE to gauge whether or not it adversely affects their mental and/or motor performance. Advise patients who require concomitant treatment with morphine to tell their prescriber if they develop signs of CNS depression such as somnolence. If this occurs the dose of GRALISE or morphine should be reduced accordingly.
- Advise patients that if they miss a dose of GRALISE to take it with food as soon as they remember. If it is almost time for the next dose, just skip the missed dose and take the next dose at the regular time. Do not take two doses at the same time.
- Advise patients that if they take too much GRALISE, to call their healthcare provider or poison control center, or go to the nearest emergency room right away.
Suicidal Thoughts and Behavior
Counsel patients, their caregivers, and families that AEDs, including gabapentin, the active ingredient in GRALISE, may increase the risk of suicidal thoughts and behavior and of the need to be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Instruct patients, caregivers, and families to report behaviors of concern immediately to healthcare providers. Also inform patients who plan to or have discontinued GRALISE that suicidal thoughts and behavior can appear even after the drug is stopped [see Warnings and Precautions (5.1)].
Respiratory Depression
Inform patients about the risk of respiratory depression. Include information that the risk is greatest for those using concomitant central nervous system (CNS) depressants (such as opioid analgesics) or in those with underlying respiratory impairment. Teach patients how to recognize respiratory depression and advise them to seek medical attention immediately if it occurs [see Warnings and Precautions (5.3)].
Dosing and Administration
GRALISE is not substitutable with other gabapentin products because of differing pharmacokinetic profiles that affect the frequency of administration.
The safety and effectiveness of GRALISE in patients with epilepsy has not been studied.
Advise patients that GRALISE should be taken orally once daily with the evening meal. GRALISE tablets should be swallowed whole. Do not split, crush, or chew the tablets [see Dosage and Administration (2.1)].
Use in Pregnancy
Advise patients to notify their healthcare provider if they become pregnant or intend to become pregnant during treatment with GRALISE, and to notify their physician if they are breast feeding or intend to breast feed during therapy [see Use in Specific Populations (8.1) and (8.2)].
Encourage patients to enroll in the North American Antiepileptic Drug (NAAED) PregnancyRegistry if they become pregnant. This registry is collecting information about the safety ofantiepileptic drugs during pregnancy. To enroll, patients can call the toll-free number 1-888-233-2334 [see Use in Specific Populations (8.1)].
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
MEDICATION GUIDE
GRALISE® (gra leez')
(gabapentin) Tablets
Read this Medication Guide before you start taking GRALISE and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment. If you have any questions about GRALISE, ask your healthcare provider or pharmacist.
What is the most important information I should know about GRALISE?Do not stop taking GRALISE without first talking with your healthcare provider. Stopping GRALISE suddenly can cause serious problems.
Like other antiepileptic drugs, gabapentin, the active ingredient in GRALISE, may cause suicidal thoughts or actions in a very small number of people, about 1 in 500. This can happen while you take GRALISE or after stopping. However, it is not known if GRALISE is safe and effective in people with seizure problems (epilepsy). Therefore, GRALISE should not be used in place of other gabapentin products.
Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempts to commit suicide
- serious breathing problems
- new or worse depression
- new or worse anxiety
- feeling agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
- Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
Serious breathing problems
- Serious breathing problems can occur when GRALISE is taken with other medicines that can cause severe sleepiness or decreased awareness, or when it is taken by someone who already has breathing problems. Watch for increased sleepiness or decreased breathing when starting GRALISE or when the dose is increased. Get help right away if breathing problems occur.
Do not stop taking GRALISE without first talking with your healthcare provider.
- Stopping GRALISE suddenly can cause serious problems.
What is GRALISE?
GRALISE is a prescription medicine used in adults, 18 years and older, to treat:
- pain from damaged nerves (neuropathic pain) that follows healing of shingles (a painful rash that comes after a herpes zoster infection).
It is not known if GRALISE is safe and effective in people with seizure problems (epilepsy).
It is not known if GRALISE is safe and effective in children under 18 years of age with postherpetic pain.
GRALISE is not substitutable with other gabapentin products.
Who should not take GRALISE?Do not take GRALISE if you are allergic to gabapentin or any of the ingredients in GRALISE. See the end of this Medication Guide for a complete list of ingredients in GRALISE.
What should I tell my healthcare provider before taking GRALISE?Before taking GRALISE, tell your healthcare provider if you:
- have or have had depression, mood problems or suicidal thoughts or behavior
- have breathing problems
- have seizures
- have kidney problems or get kidney dialysis
-
are pregnant or plan to become pregnant. It is not known if GRALISE can harm your unborn baby. Tell your healthcare provider right away if you become pregnant while taking GRALISE. You and your healthcare provider will decide if you should take GRALISE while you are pregnant.
- If you become pregnant while taking GRALISE, talk to your healthcare provider about registering with the North American Antiepileptic Drug (NAAED) Pregnancy Registry. The purpose of this registry is to collect information about the safety of antiepileptic drugs, including gabapentin, the active ingredient in GRALISE, during pregnancy. You can enroll in this registry by calling 1-888-233-2334.
- are breastfeeding or plan to breastfeed. Gabapentin passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby during treatment with GRALISE.
Tell your healthcare provider about all the medicines you take including prescription and non-prescription medicines, vitamins or herbal supplements. Especially tell your healthcare provider if you take any opioid pain medicine (such as oxycodone), or medicines for anxiety (such as lorazepam) or insomnia (such as zolpidem). You may have a higher chance for dizziness, sleepiness, or serious breathing problems if these medicines are taken with GRALISE.
Taking GRALISE with certain other medicines can cause side effects or affect how well they work. Do not start or stop other medicines without talking to your healthcare provider.
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take GRALISE?- Take GRALISE exactly as prescribed. Your healthcare provider will tell you how much GRALISE to take and when to take it. Take GRALISE at the same time each day.
- Do not change your dose or stop taking GRALISE without talking with your healthcare provider. If you stop taking GRALISE suddenly, you may experience side effects. Talk with your healthcare provider about how to stop GRALISE slowly.
- Take GRALISE with food one time each day with your evening meal.
- Take GRALISE tablets whole. Do not split, crush, or chew GRALISE tablets before swallowing.
- Your healthcare provider may change your dose of GRALISE. Do not change your dose of GRALISE without talking to your healthcare provider.
- If you miss a dose, take it as soon as you remember with food. If it is almost time for your next dose, just skip the missed dose. Take the next dose at your regular time. Do not take two doses at the same time.
- If you take too much GRALISE, call your healthcare provider or poison control center, or go to the nearest emergency room right away.
- If you are taking an antacid containing aluminum hydroxide and magnesium hydroxide, it is recommended that GRALISE be taken at least 2 hours following administration of the antacid.
What should I avoid while taking GRALISE?- Do not drink alcohol or take other medicines that make you sleepy or dizzy while taking GRALISE without first talking to your healthcare provider. Taking GRALISE with alcohol or medicines that cause sleepiness or dizziness may make your sleepiness or dizziness worse.
- Do not operate heavy machines or do other dangerous activities until you know how GRALISE affects you. GRALISE can slow your thinking and motor skills.
What are the possible side effects of GRALISE?
The most common side effect of GRALISE is:
- dizziness
Tell your healthcare provider about any side effect that bothers you or that does not go away.
These are not all the possible side effects of GRALISE. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store GRALISE?
Store GRALISE at 59°F to 86°F (15°C to 30°C)
- Keep GRALISE and all medicines out of the reach of children.
General information about the safe and effective use of GRALISE
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use GRALISE for a condition for which it was not prescribed. Do not give GRALISE to other people, even if they have the same symptoms you have. It may harm them.
This Medication Guide summarizes the most important information about GRALISE. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about GRALISE that is written for health professionals.
For more information about GRALISE, call 1-877-447-7979.
What are the ingredients in GRALISE?
Active ingredient: gabapentin
Inactive ingredients:
300 mg tablet: copovidone, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene oxide, and white film coating (polyvinyl alcohol, titanium dioxide, talc, polyethylene glycol 3350, and lecithin (soya)).
450 mg tablet: copovidone, hypromellose, magnesium stearate, polyethylene oxide, and red film coating (polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide, and iron oxide red).
600 mg tablet: copovidone, hypromellose, magnesium stearate, polyethylene oxide, and beige film coating (polyvinyl alcohol, titanium dioxide, talc, polyethylene glycol 3350, iron oxide yellow, and iron oxide red).
750 mg tablet: copovidone, hypromellose, magnesium stearate, polyethylene oxide, and yellow film coating (polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide, and iron oxide yellow).
900 mg tablet: copovidone, hypromellose, magnesium stearate, polyethylene oxide, and pink film coating (polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide, and iron oxide red).
Distributed by:
Almatica Pharma LLC
Morristown, NJ 07960 USABrands listed are trademarks of their respective owners.
PL803-02
Revised: 05/2025This Medication Guide has been approved by the U.S. Food and Drug Administration.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GRALISE
gabapentin tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 52427-803 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GABAPENTIN (UNII: 6CW7F3G59X) (GABAPENTIN - UNII:6CW7F3G59X) GABAPENTIN 300 mg Inactive Ingredients Ingredient Name Strength COPOVIDONE K25-31 (UNII: D9C330MD8B) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TALC (UNII: 7SEV7J4R1U) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) Product Characteristics Color white Score no score Shape OVAL Size 16mm Flavor Imprint Code SLV;300 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52427-803-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022544 10/01/2020 GRALISE
gabapentin tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 52427-804 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GABAPENTIN (UNII: 6CW7F3G59X) (GABAPENTIN - UNII:6CW7F3G59X) GABAPENTIN 450 mg Inactive Ingredients Ingredient Name Strength COPOVIDONE K25-31 (UNII: D9C330MD8B) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TALC (UNII: 7SEV7J4R1U) FERRIC OXIDE RED (UNII: 1K09F3G675) POLYETHYLENE OXIDE 7000000 (UNII: G3MS6M810Y) Product Characteristics Color RED Score no score Shape OVAL Size 17mm Flavor Imprint Code ALM;450 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52427-804-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 12/31/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022544 12/31/2023 GRALISE
gabapentin tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 52427-806 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GABAPENTIN (UNII: 6CW7F3G59X) (GABAPENTIN - UNII:6CW7F3G59X) GABAPENTIN 600 mg Inactive Ingredients Ingredient Name Strength COPOVIDONE K25-31 (UNII: D9C330MD8B) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TALC (UNII: 7SEV7J4R1U) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) Product Characteristics Color BROWN (beige) Score no score Shape OVAL Size 19mm Flavor Imprint Code SLV;600 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52427-806-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022544 10/01/2020 GRALISE
gabapentin tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 52427-850 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GABAPENTIN (UNII: 6CW7F3G59X) (GABAPENTIN - UNII:6CW7F3G59X) GABAPENTIN 750 mg Inactive Ingredients Ingredient Name Strength COPOVIDONE K25-31 (UNII: D9C330MD8B) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TALC (UNII: 7SEV7J4R1U) POLYETHYLENE OXIDE 7000000 (UNII: G3MS6M810Y) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color YELLOW Score no score Shape OVAL Size 19mm Flavor Imprint Code ALM;750 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52427-850-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 12/31/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022544 12/31/2023 GRALISE
gabapentin tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 52427-890 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GABAPENTIN (UNII: 6CW7F3G59X) (GABAPENTIN - UNII:6CW7F3G59X) GABAPENTIN 900 mg Inactive Ingredients Ingredient Name Strength COPOVIDONE K25-31 (UNII: D9C330MD8B) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TALC (UNII: 7SEV7J4R1U) FERRIC OXIDE RED (UNII: 1K09F3G675) POLYETHYLENE OXIDE 7000000 (UNII: G3MS6M810Y) Product Characteristics Color PINK Score no score Shape OVAL Size 20mm Flavor Imprint Code ALM;900 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52427-890-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 12/31/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022544 12/31/2023 GRALISE
gabapentin kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 52427-815 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52427-815-99 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 10/01/2020 08/01/2024 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 9 Part 2 24 Part 1 of 2 GRALISE
gabapentin tablet, film coatedProduct Information Item Code (Source) NDC: 52427-803 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GABAPENTIN (UNII: 6CW7F3G59X) (GABAPENTIN - UNII:6CW7F3G59X) GABAPENTIN 300 mg Inactive Ingredients Ingredient Name Strength COPOVIDONE K25-31 (UNII: D9C330MD8B) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TALC (UNII: 7SEV7J4R1U) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) Product Characteristics Color white Score no score Shape OVAL Size 16mm Flavor Imprint Code SLV;300 Contains Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022544 10/01/2020 Part 2 of 2 GRALISE
gabapentin tablet, film coatedProduct Information Item Code (Source) NDC: 52427-806 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GABAPENTIN (UNII: 6CW7F3G59X) (GABAPENTIN - UNII:6CW7F3G59X) GABAPENTIN 600 mg Inactive Ingredients Ingredient Name Strength COPOVIDONE K25-31 (UNII: D9C330MD8B) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TALC (UNII: 7SEV7J4R1U) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) Product Characteristics Color BROWN (beige) Score no score Shape OVAL Size 19mm Flavor Imprint Code SLV;600 Contains Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022544 10/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022544 10/01/2020 08/01/2024 Labeler - Almatica Pharma LLC (962454505)
Trademark Results [Gralise]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 GRALISE 88511419 not registered Live/Pending |
Assertio Therapeutics, Inc. 2019-07-12 |
 GRALISE 86982952 5312458 Live/Registered |
ASSERTIO THERAPEUTICS, INC. 2016-01-14 |
 GRALISE 86875330 not registered Dead/Abandoned |
ASSERTIO THERAPEUTICS, INC. 2016-01-14 |
 GRALISE 77934261 4132334 Live/Registered |
ASSERTIO THERAPEUTICS, INC. 2010-02-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.





