INFANTS GAS DROPS- simethicone suspension/ drops
Infants Gas Drops by
Drug Labeling and Warnings
Infants Gas Drops by is a Otc medication manufactured, distributed, or labeled by Rij Pharmaceutical Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
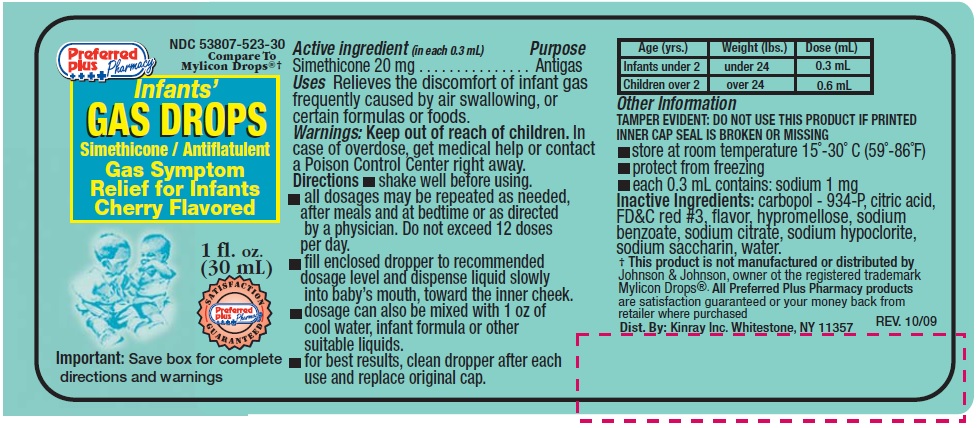
- Active ingredients (in each 0.3mL)
- Purpose
- Uses
- Warnings
-
Directions
- shake well before using
- all dosages may be repeated as needed, after meals and at bedtime or as directed by a physician. Do not exceed 12 doses per day.
- fill enclosed dropper to recommended dosage level and dispense liquid slowly into baby's mouth, toward the inner cheek
- dosage can also be mixed with 1 oz.of cool water, infant formula or other suitable liquids
- for best results, clean dropper after each use and replace original cap.
Age (yrs) Weight (lbs) Dose (ml) Infants under 2 Under 24 0.3 mL Children over 2 Over 24 0.6 mL - Other Information
- Inactive ingredients
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
INFANTS GAS DROPS
simethicone suspension/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 53807-523 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Dimethicone (UNII: 92RU3N3Y1O) (Dimethicone - UNII:92RU3N3Y1O) Dimethicone 20 mg in 0.3 mL Inactive Ingredients Ingredient Name Strength SODIUM (UNII: 9NEZ333N27) CARBOMER HOMOPOLYMER TYPE B (ALLYL SUCROSE CROSSLINKED) (UNII: Z135WT9208) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) HYPROMELLOSES (UNII: 3NXW29V3WO) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM HYPOCHLORITE (UNII: DY38VHM5OD) SACCHARIN SODIUM (UNII: SB8ZUX40TY) WATER (UNII: 059QF0KO0R) Product Characteristics Color PINK Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 53807-523-30 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/16/1996 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part332 03/16/1996 Labeler - Rij Pharmaceutical Corporation (144679156) Establishment Name Address ID/FEI Business Operations Rij Pharmaceutical Corporation 144679156 manufacture(53807-523)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.