COLD ARMOR (allium cepa, hydrastis canadensis, pulsatilla- pratensis, dulcamara, nux vomica, ferrum phosphoricum, gelsemium sempervirens, kali bichromicum spray
Cold Armor by
Drug Labeling and Warnings
Cold Armor by is a Homeopathic medication manufactured, distributed, or labeled by Ratis, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
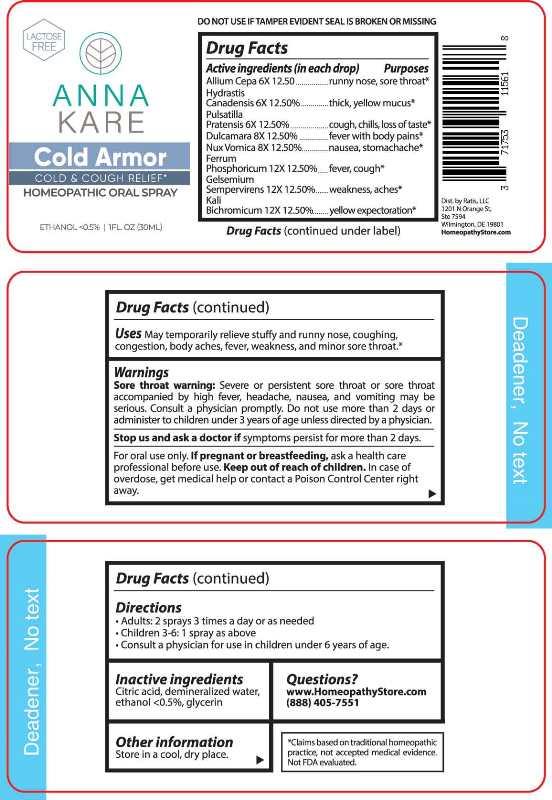
- ACTIVE INGREDIENTS:
-
PURPOSES:
Allium Cepa – runny nose, sore throat,* Hydrastis Canadensis – thick , yellow mucus,* Pulsatilla Pratensis – cough, chills, loss of taste,* Dulcamara – fever with body pains,* Nux Vomica – nausea, stomachache,* Ferrum Phosphoricum – fever, cough,* Gelsemium Sempervirens – weakness, aches,* Kali Bichromicum – yellow expectoration.*
- USES:
-
WARNINGS:
Sore throat warning: Severe or persistent sore throat or sore throat accompanied by high fever, headache, nausea, and vomiting may be serious. Consult a physician promptly. Do not use more than 2 days or administer to children under 3 years of age unless directed by a physician.
Stop use and ask a doctor if symptoms persist for more than 2 days.
For oral use only. If pregnant or breast-feeding, ask a health care professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control center right away.
DO NOT USE IF TAMPER EVIDENT SEAL IS BROKEN OR MISSING
- KEEP OUT OF REACH OF CHILDREN:
- DIRECTIONS:
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE LABLE DISPLAY:
-
INGREDIENTS AND APPEARANCE
COLD ARMOR
allium cepa, hydrastis canadensis, pulsatilla (pratensis), dulcamara, nux vomica, ferrum phosphoricum, gelsemium sempervirens, kali bichromicum sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 71753-1156 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ONION (UNII: 492225Q21H) (ONION - UNII:492225Q21H) ONION 6 [hp_X] in 1 mL GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 6 [hp_X] in 1 mL PULSATILLA PRATENSIS WHOLE (UNII: 8E272251DI) (PULSATILLA PRATENSIS WHOLE - UNII:8E272251DI) PULSATILLA PRATENSIS WHOLE 6 [hp_X] in 1 mL SOLANUM DULCAMARA TOP (UNII: KPS1B1162N) (SOLANUM DULCAMARA TOP - UNII:KPS1B1162N) SOLANUM DULCAMARA TOP 8 [hp_X] in 1 mL STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 8 [hp_X] in 1 mL FERROSOFERRIC PHOSPHATE (UNII: 91GQH8I5F7) (FERROSOFERRIC PHOSPHATE - UNII:91GQH8I5F7) FERROSOFERRIC PHOSPHATE 12 [hp_X] in 1 mL GELSEMIUM SEMPERVIRENS ROOT (UNII: 639KR60Q1Q) (GELSEMIUM SEMPERVIRENS ROOT - UNII:639KR60Q1Q) GELSEMIUM SEMPERVIRENS ROOT 12 [hp_X] in 1 mL POTASSIUM DICHROMATE (UNII: T4423S18FM) (DICHROMATE ION - UNII:9LKY4BFN2V) POTASSIUM DICHROMATE 12 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71753-1156-1 30 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 05/26/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/26/2023 Labeler - Ratis, LLC (964594324)
Trademark Results [Cold Armor]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 COLD ARMOR 78969907 not registered Dead/Abandoned |
Northern Wilderness Outfitters 2006-09-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.