OPTISON PERFLUTREN PROTEIN-TYPE A MICROSPHERES- human albumin microspheres and perflutren injection, solution
Optison by
Drug Labeling and Warnings
Optison by is a Prescription medication manufactured, distributed, or labeled by GE Healthcare Inc., GE Healthcare AS, Octapharma Pharmazeutika Produktionsgesellschaft, m.b.H, Octapharma AB. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use OPTISON safely and effectively. See full prescribing information for OPTISON.
OPTISON (perflutren protein-type A microspheres) injectable suspension, for intravenous use
Initial U.S. Approval: 1997WARNING: SERIOUS CARDIOPULMONARY REACTIONS
See full prescribing information for complete boxed warning.
Serious cardiopulmonary reactions, including fatalities, have occurred uncommonly during or following perflutren-containing microsphere administration. Most serious reactions occur within 30 minutes of administration (5.1).
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Optison is an ultrasound contrast agent indicated for use in patients with suboptimal echocardiograms to opacify the left ventricle and to improve the delineation of the left ventricular endocardial borders. (1)
DOSAGE AND ADMINISTRATION
Recommended Dosage (2.1)
- Recommended dose of Optison is 0.5 mL injected into a peripheral vein
- Maximum total dose should not exceed 5.0 mL in any 10 minute period
- Maximum total dose should not exceed 8.7 mL in any one patient study
- If contrast enhancement is inadequate after the dose of 0.5 mL, additional doses of increments of 0.5 mL up to 5.0 mL in a 10 minutes period may be injected intravenously up to a maximum total dose of 8.7 mL.
Administration Instructions (2.3)
- For intravenous injection. Do not administer by intra-arterial injection (5.3)
- Injection rate should not exceed 1 mL per second
- Follow the Optison injection with a flush of 0.9% Sodium Chloride Injection, USP, or 5% Dextrose Injection, USP
DOSAGE FORMS AND STRENGTHS
Injectable suspension: 5.0-8.0×108 protein-type A microspheres, 10 mg albumin human, and 0.22 ± 0.11 mg, perflutren per mL in 3mL single-patient use vials (3).
CONTRAINDICATIONS
- Do not administer Optison to patients with known or suspected hypersensitivity to perflutren, blood, blood products or albumin (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Most common adverse reactions during treatment: headache, nausea and/or vomiting, warm sensation or flushing, and dizziness (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact GE Healthcare at 1-800-654-0118 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 1/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: SERIOUS CARDIOPULMONARY REACTIONS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Preparation Instructions
2.3 Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serious Cardiopulmonary Reactions
5.2 Hypersensitivity Reactions
5.3 Systemic Embolization
5.4 Ventricular Arrhythmia Related to High Mechanical Index
5.5 Transmissible Infectious Agents
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Echocardiography
14.2 Pulmonary Hemodynamic Effects
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: SERIOUS CARDIOPULMONARY REACTIONS
Serious cardiopulmonary reactions, including fatalities, have occurred uncommonly during or following perflutren-containing microsphere administration. Most serious reactions occur within 30 minutes of administration [see Warnings and Precautions (5.1)].
- Assess all patients for the presence of any condition that precludes OPTISON administration [see Contraindications (4)].
- Always have resuscitation equipment and trained personnel readily available [see Warnings and Precautions (5.1)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
- The recommended dose of Optison is 0.5 mL injected into a peripheral vein.
- If the contrast enhancement is inadequate after the dose of 0.5 mL, additional doses in increments of 0.5 mL may be repeated for further contrast enhancement as needed.
- The maximum total dose should not exceed 5mL in any 10 minute period.
- The maximum total dose should not exceed 8.7 mL in any one patient study.
2.2 Preparation Instructions
- Do not use if the container has been damaged, the protective seal and/or rubber cap have been entered, or the upper white layer is absent (may indicate the microspheres have been damaged and may result in poor or no echo contrast).
- Invert the Optison vial and gently rotate to resuspend the microspheres. This process will allow the product to come to room temperature before use.
- Inspect the vial for complete resuspension. Do not use if the solution appears to be clear rather than opaque and milky-white.
- Vent the Optison vial with a sterile vent spike or with a sterile 18 gauge needle before withdrawing the Optison suspension into the injection syringe.
- Do not inject air into the vial.
- Use the product within one minute of suspension. If one minute is exceeded, resuspend by inverting and gently rotating the microsoheres in the syringe. Failure to adequately resuspend Optison may cause inadequate delivery of the microspheres, and may result in inadequate contrast.
2.3 Administration Instructions
- Inspect visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not inject if the solution is not opaque, milky-white, and absent particulate matter.
- Inject through a 20-gauge or larger angiocatheter into a peripheral vein at a rate not exceeding 1 mL per second. Suggested methods of administration include: a short extension tubing, heparin lock, or intravenous line, all with a 3-way stopcock.
- Administer intravenously; do not administer Optison by intra-arterial injection [see Warnings and Precautions (5.3)].
- Do not aspirate blood back into the Optison containing syringe before administration; this may promote the formation of a blood clot within the syringe.
- For short extension tubing or heparin lock: fill one syringe with 0.9% Sodium Chloride Injection, USP, and FLUSH the line for patency before and after the injection of Optison.
- For a continuous intravenous line: open an intravenous line with 0.9% Sodium Chloride Injection, USP (or 5% Dextrose Injection, USP) at a slow infusion rate to maintain vascular patency. Flush the line immediately after injection of Optison
- Do not use the single-patient use vial for more than one patient. Discard unused product.
-
3 DOSAGE FORMS AND STRENGTHS
Injectable suspension: 3 mL single-patient use vial containing a clear liquid lower layer and a white liquid upper layer, and a headspace filled with perflutren gas. Each mL of Optison contains 5-8×108 protein-type A microspheres, 10 mg albumin human, and 0.22 ± 0.11 mg perflutren. The sterile suspension is homogeneous, opaque, and milky-white after resuspension.
-
4 CONTRAINDICATIONS
Do not administer Optison to patients with known or suspected hypersensitivity to perflutren, blood, blood products or albumin [see Warnings and Precautions (5.5)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Serious Cardiopulmonary Reactions
Serious cardiopulmonary reactions including fatalities have occurred uncommonly during or shortly following perflutren-containing microsphere administration, typically within 30 minutes of administration. The risk for these reactions may be increased among patients with unstable cardiopulmonary conditions (acute myocardial infarction, acute coronary artery syndromes, worsening or unstable congestive heart failure, or serious ventricular arrhythmias).
The reported reactions to perflutren-containing microspheres include: fatal cardiac or respiratory arrest, shock, syncope, symptomatic arrhythmias (atrial fibrillation, tachycardia, bradycardia, supraventricular tachycardia, ventricular fibrillation, ventricular tachycardia), hypertension, hypotension, dyspnea, hypoxia, chest pain, respiratory distress, stridor, wheezing, loss of consciousness and convulsions [see Adverse Reactions (6.2)].
Always have cardiopulmonary resuscitation personnel and equipment readily available prior to Optison administration and monitor all patients for acute reactions.
5.2 Hypersensitivity Reactions
Serious anaphylactic reactions have been observed during or shortly following perflutren-containing microsphere administration including: Shock, hypersensitivity, bronchospasm, throat tightness, angioedema, edema (pharyngeal, palatal, mouth, peripheral, localized), swelling (face, eye, lip, tongue upper airway), facial hypoesthesia, rash, urticaria, pruritus, flushing, and erythema have occurred in patients with no prior exposure to perflutren-containing microsphere products. Always have cardiopulmonary resuscitation personnel and equipment readily available prior to Optison administration and monitor all patients for hypersensitivity reactions.
5.3 Systemic Embolization
When administering Optison to patients with a cardiac shunt, microspheres can bypass filtering of the lung and enter the arterial circulation. Assess patients with shunts for embolic phenomena following Optison administration. Optison is only for intravenous administration; do not administer Optison by intra-arterial injection [see Dosage and Administration (2.3)].
5.4 Ventricular Arrhythmia Related to High Mechanical Index
High ultrasound mechanical index values may cause microsphere rupture and lead to ventricular arrhythmias. Additionally, end-systolic triggering with high mechanical indices has been reported to cause ventricular arrhythmias. Optison is not recommended for use at mechanical indices greater than 0.8.
5.5 Transmissible Infectious Agents
This product contains albumin, a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral disease. A theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD) also is considered extremely remote. No cases of transmission of viral disease or CJD have ever been identified for albumin.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Serious Cardiopulmonary Reactions [see Warnings and Precautions (5.1)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Optison was administered in clinical studies in 279 patients. Of these patients there were 192 (68.8%) men and 87 (31.2%) women. The racial demographics were 199 (71.3%) Caucasian, 52 (18.6%) Black, 24 (8.6%) Hispanic, and 4 (1.4%) other racial or ethnic groups.
In these patients, 47 (16.8%) reported at least one adverse reaction. Of these one reaction was serious and required treatment with antihistamines for hypersensitivity manifestations of dizziness, nausea, flushing and temperature elevation. Deaths were not reported during the clinical studies.
Of the reported adverse reactions following the use of Optison the most frequently reported were headache (5.4%), nausea and/or vomiting (4.3%), warm sensation or flushing (3.6%), and dizziness (2.5%). The most common adverse reactions observed in clinical studies of Optison are given in Table 1.
Table 1 SELECTED ADVERSE REACTIONS REPORTED IN ≥ 0.5% OF THE SUBJECTS WHO RECEIVED OPTISON™ IN CONTROLLED CLINICAL STUDIES) (1) Patients are counted separately within each body system. (2) The body system is reported if the aggregate is ≥ 0.5%. Details are not shown if the subsystem is not ≥ 0.5%. No. of Patients Exposed to Optison 279 No. of Patients Reporting on Adverse Reactions 47 (16.8%) Body as a Whole 38 (13.6%) Headache 15 (5.4%) Warm Sensation/Flushing 10 (3.6%) Chills/fever 4 (1.4%) Flu-like Symptoms 3 (1.1%) Malaise/Weakness/Fatigue 3 (1.1%) Cardiovascular System 12 (4.3%) Dizziness 7 (2.5%) Chest Pain 3 (1.1%) Digestive System 12 (4.3%) Nausea and/or Vomiting 12 (4.3%) Nervous System 3 (1.1%) Respiratory System 5 (1.8%) Dyspnea 3 (1.1%) Skin & Appendages 11 (3.9%) Injection Site Discomfort 3 (1.1%) Erythema 2 (0.7%) Special Senses 9 (3.2%) Altered Taste 5 (1.8%) Adverse reactions reported in < 0.5% of subjects who received Optison included: arthralgia, back pain, body or muscle aches, induration, urticaria, dry mouth, palpitations, paresthesia, photophobia, premature ventricular contraction, pruritus, rash, irritableness, hypersensitivity, tinnitus, tremor, visual blurring, wheezing, oxygen saturation decline due to coughing, discoloration at the injection site, and burning sensation in the eyes.
6.2 Postmarketing Experience
In a prospective, post-marketing safety surveillance study of Optison used in routine clinical practice, a total of 1039 subjects received Optison. Of these patients, 648 (62.4%) were male and 391 (37.6%) were female with average age of 59.9 years (min, max: 20, 97). The racial distributions were 864 (83.2%) White, 141 (13.6%) Black, 18 (1.7%) Asian, and 16 (1.5%) other racial or ethnic groups. Overall, 175 patients (16.8%) reported at least one adverse event. No serious adverse reactions, including deaths, were reported in this study.
The following adverse reactions have been identified during the postmarketing use of Optison. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiac arrests and other serious but non-fatal adverse reactions were uncommonly reported. Most of these reactions included cardiopulmonary symptoms and signs such as cardiac arrest, hypotension, supraventricular and ventricular arrhythmias, respiratory distress or decreased oxygenation. Reports also identified neurologic reactions (loss of consciousness or convulsions) as well as hypersensitivity reactions [see Warnings and Precautions (5.2)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data with Optison use in pregnant women to inform any drug-associated risks. No adverse developmental outcomes were observed in animal reproduction studies with intravenous administration of Optison to pregnant rats and rabbits during organogenesis at doses up to at least 5 and 10 times the recommended human dose based on body surface area (see Data).
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
Optison was administered intravenously to rats at doses of 0.25, 5 and 10 mL/kg/day (approximately 0.2, 5 and 10 times the recommended maximum human dose of 8.7 mL, respectively, based on body surface area) and to rabbits at 0.25, 2.5 and 5 mL/kg/day (approximately 0.5, 5 and 10 times the recommended maximum human dose, respectively, based on body surface area) during organogenesis. No significant findings attributable solely to a direct effect on the fetus were detected in the studies.
8.2 Lactation
There are no data on the presence of perflutren protein-type A microspheres in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Optison and any potential adverse effects on the breastfed infant from Optison or from the underlying maternal condition.
8.5 Geriatric Use
Of the total number of subjects in a clinical study of Optison, 35% were 65 and over, while 14 % were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
-
11 DESCRIPTION
Optison (perflutren protein-type A microspheres) injectable suspension is an ultrasound contrast agent for intravenous injection. The vial contains a clear liquid lower layer, a white liquid upper layer, and a headspace filled with perflutren gas. After resuspension, the sterile suspension is homogeneous, opaque, and milky-white.
Perflutren is chemically characterized as 1,1,1,2,2,3,3,3-perflutren with a molecular weight of 188, an empirical formula of C3F8 and it has the following structural formula:
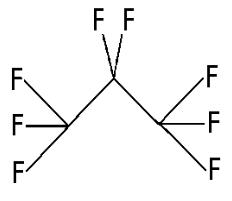
Each mL of Optison contains 5.0-8.0×108 protein-type A microspheres, 10 mg albumin human, 0.22 ± 0.11 mg/mL perflutren; and the following excipients: 0.2 mg N-acetyltryptophan, and 0.12 mg caprylic acid in 0.9% aqueous sodium chloride. The headspace of the vial is filled with perflutren gas. The pH is adjusted to 6.4-7.4. The protein in the microsphere shell makes up approximately 5-7% (w/w) of the total protein in the suspension. The microsphere particle size parameters are listed in Table 2.
Table 2 Microsphere Particle Size Parameters Mean diameter (range) 3.0-4.5 µm (max. 32.0µm) Percent less than 10µm 95% -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The Optison microspheres create an echogenic contrast effect in the blood. The acoustic impedance of the Optison microspheres is much lower than that of the blood. Therefore, impinging ultrasound waves are scattered and reflected at the microsphere-blood interface and ultimately may be visualized in the ultrasound image. At the frequencies used in adult echocardiography (2-5 MHz), the microspheres resonate which further increases the extent of ultrasound scattering and reflection.
12.2 Pharmacodynamics
The median duration of Optison contrast enhancement for each of the four doses of Optison, 0.2 (40% of recommended dose), 0.5, 3.0, and 5 mL , were approximately one, two, four, and five minutes, respectively [see Clinical Studies (14.1)].
12.3 Pharmacokinetics
After injection of Optison, diffusion of the perflutren gas out of the microspheres is limited by the low partition coefficient of the gas in blood that contributes to the persistence of the microspheres.
The pharmacokinetics of the intact microspheres of Optison in humans are unknown.
Distribution
The binding of perflutren to plasma proteins and its partitioning into blood cells are unknown. However, perflutren protein binding is expected to be minimal due to the low partition coefficient of the gas in blood.
Elimination
Following intravenous injection, perflutren is cleared with a pulmonary elimination half-life of 1.3 ± 0.69 minutes (mean ± SD).
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Animal studies were not carried out to determine the carcinogenic potential of Optison.
Mutagenesis
The result of the following genotoxicity studies with Optison were negative: 1) Salmonella/Escherichia coli reverse mutation assay, 2) in vitro mammalian chromosome aberration assay using Chinese hamster ovary cells (CHO) with and without metabolic activation, 3) CHO/HGPRT forward mutation assay, and 4) in vivo mammalian micronucleus assay.
-
14 CLINICAL STUDIES
14.1 Echocardiography
The efficacy of Optison was evaluated in two identical multicenter, controlled, dose escalation studies of 203 patients (Study A: n=101, Study B: n=102) with sub-optimal non-contrast echocardiography defined as having at least two out of six segments of the left ventricular endocardial border inadequately delineated in the apical four-chamber view. Among these patients there were 79% men, 21% women, 64% White, 25% Black, 10% Hispanic, and 1% other race or ethnic group. The patients had a mean age of 61 years (range: 21 to 83 years), a mean weight of 196 lbs. (range: 117 to 342 lbs.), a mean height of 68 inches (range: 47 to 78 inches), and a mean body surface area of 2.0m2 (range: 1.4 to 2.6m2). Approximately 23% of the patients had chronic pulmonary disease, and 17% had congestive and dilated cardiomyopathy with left ventricular ejection fractions (LVEFs) of between 20% and 40% (by previous echocardiography). Patients with a LVEF of less than 20% or with New York Heart Association Class IV heart failure were not included in the studies.
After non-contrast imaging, Optison was administered in increasing increments as 4 doses (0.2, 0.5, 3.0 and 5 mL) with at least ten minutes between each dose. Ultrasound settings were optimized for the baseline (non-contrast) apical four-chamber view and remained unchanged for the contrast imaging. Static echocardiographic images and video-tape segments were interpreted by a reader who was blinded to the patient's clinical history and to the dose of Optison. Left ventricular endocardial border delineation and left ventricular opacification, were assessed before and after Optison administration by the measurement of visualized endocardial border length and ventricular opacification.
In comparison to non-contrast ultrasound, Optison significantly increased the length of endocardial border that could be visualized both at end-systole and end-diastole (see Table 3). In these patients there was a trend towards less visualization in women. Optison increased left ventricular opacification (peak intensity) in the mid-chamber and apical views (see Table 4). The imaging effects of Optison on endocardial border delineation and left ventricular opacification were similar at doses between 0.5 ml and 5 ml and were also similar among patients with or without pulmonary disease and dilated cardiomyopathy.
Table 3 Left Ventricular Endocardial Border Length Before and After OPTISON *, † Length at End-Systole (cm) Length at End-Diastole (cm) OPTISON dose n mean ± S.D. n mean - * The differences in the number of enrolled patients and evaluated patients at each dose reflects exclusions based on withdrawal from the trial, or those with technically inadequate or missing images.
- † An intent-to-treat analysis, with non-favorable values imputed for missing patients, provided qualitatively similar results.
Study A (n=101) 0 mL (baseline) 87 7.7 ± 3.0 86 9.3 ± 3.4 0.5 mL 86 12.0 ± 4.9 91 15.8 ± 5.1 Study B (n=102) 0 mL (baseline) 89 8.1 ± 3.4 89 9.6 ± 3.7 0.5 mL 95 12.4 ± 4.9 97 16.4 ± 4.6 Table 4 Intensity of Left Ventricular Opacification* Before and After OPTISON™ †,‡ Mid-Chamber Apex Intensity at End-Diastole Intensity at End-Systole Intensity at End-Diastole Intensity at End-Systole OPTISON dose n mean ± S.D. n mean ± S.D. n mean ± S.D. n mean ± S.D. - * Intensity measured by video densitometry in arbitrary gray scale units (0-255).
- † The differences in the number of enrolled patients and evaluated patients at each dose reflects exclusions based on withdrawal from the trial, or those with technically inadequate or missing images.
- ‡ An intent-to-treat analysis, with non-favorable values imputed for missing patients, provided qualitatively similar results.
Study A (n = 101) 0 mL (baseline) 91 39.5 ± 16.9 91 40.0 ± 18.1 91 46.7 ± 19.7 91 46.9 ± 20.1 0.5 mL 91 57.3 ± 26.8 90 57.4 ± 26.7 91 67.0 ± 30.1 90 64.1 ± 30.2 Study B (n = 102) 0 mL (baseline) 95 40.4 ± 17.4 95 40.9 ± 17.5 95 43.7 ± 19.9 95 45.0 ± 19.6 0.5 mL 97 53.3 ± 20.7 96 53.6 ± 21.0 97 64.4 ± 25.3 96 61.6 ± 26.7 14.2 Pulmonary Hemodynamic Effects
The effect of Optison on pulmonary hemodynamics was studied in a prospective, open-label study of 30 patients scheduled for pulmonary artery catheterization, including 19 with an elevated baseline pulmonary arterial systolic pressure (PASP) (>35 mmHg) and 11 with a normal PASP (≤35 mmHg). Systemic hemodynamic parameters and ECGs were also evaluated. No clinically important pulmonary hemodynamic, systemic hemodynamic, or ECG changes were observed. This study did not assess the effect of Optison on visualization of cardiac or pulmonary structures.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Optison is supplied as 3 mL single-patient use vials containing a clear liquid lower layer, a white liquid upper layer, and a headspace filled with perflutren gas and is homogeneous, opaque, and milky-white after resupsension. Each mL contains 5-8 ×108 protein-type A microspheres, 10 mg albumin human, and 0.22 ± 0.11 mg perflutren:
- Five (5) – 3 mL vials per carton
- Eighteen (18) – 3mL vials per carton
NDC: 0407-2707-03
NDC: 0407-2707-18 - 17 PATIENT COUNSELING INFORMATION
-
SPL UNCLASSIFIED SECTION
Distributed by GE Healthcare Inc., Marlborough, MA 01752 U.S.A.
Manufactured by GE Healthcare AS, Oslo, Norway
OPTISON™ is a trademark of GE Healthcare or one of its subsidiaries
GE and the GE Monogram are trademarks of General Electric Company.
Product of Norwegian Origin.© 2017 General Electric Company - All rights reserved.
OPT-1I-OSLO
Revised January 2017 -
PRINCIPAL DISPLAY PANEL - 3 mL Vial Carton Label
GE Healthcare
NDC: 0407-2707-18
Rx ONLY2707-18
Contains
18 x 3 mL VialsOPTISON™
(Perflutren Protein-Type A
Microspheres Injectable
Suspension, USP)3 mL
EXP.:
LOT:

-
INGREDIENTS AND APPEARANCE
OPTISON PERFLUTREN PROTEIN-TYPE A MICROSPHERES
human albumin microspheres and perflutren injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0407-2707 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Human Albumin Microspheres (UNII: T8C6W1N6NW) (Human Albumin Microspheres - UNII:T8C6W1N6NW) Human Albumin Microspheres 10 mg in 1 mL Perflutren (UNII: CK0N3WH0SR) (Perflutren - UNII:CK0N3WH0SR) Perflutren 0.22 mg in 1 mL Inactive Ingredients Ingredient Name Strength Acetyltryptophan, Dl- (UNII: 4460NBV53F) 0.2 mg in 1 mL CAPRYLIC ACID (UNII: OBL58JN025) 0.12 mg in 1 mL Sodium Chloride (UNII: 451W47IQ8X) Product Characteristics Color WHITE (Clear lower layer and white upper layer) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0407-2707-03 5 in 1 CARTON 01/02/2002 1 3 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product 2 NDC: 0407-2707-18 18 in 1 CARTON 01/02/2002 2 3 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020899 01/02/2002 Labeler - GE Healthcare Inc. (053046579) Establishment Name Address ID/FEI Business Operations Mallinckrodt Inc. 557570652 ANALYSIS(0407-2707) , API MANUFACTURE(0407-2707) , MANUFACTURE(0407-2707) Establishment Name Address ID/FEI Business Operations GE Healthcare AS 515048908 MANUFACTURE(0407-2707) , RELABEL(0407-2707) , REPACK(0407-2707)
Trademark Results [Optison]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 OPTISON 87307507 5241594 Live/Registered |
ONDA CORPORATION 2017-01-19 |
 OPTISON 87307446 5265616 Live/Registered |
ONDA Corporation 2017-01-19 |
 OPTISON 78146447 2881329 Dead/Cancelled |
GE HEALTHCARE AS 2002-07-23 |
 OPTISON 75066523 2160819 Live/Registered |
GE HEALTHCARE AS 1996-03-01 |
 OPTISON 74388569 1947178 Dead/Cancelled |
Zanelli, Claudio 1993-05-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.