BRIGHT SOLUTIONS ANTISEPTIC- pcmx, hand soap soap
Bright Solutions antiseptic by
Drug Labeling and Warnings
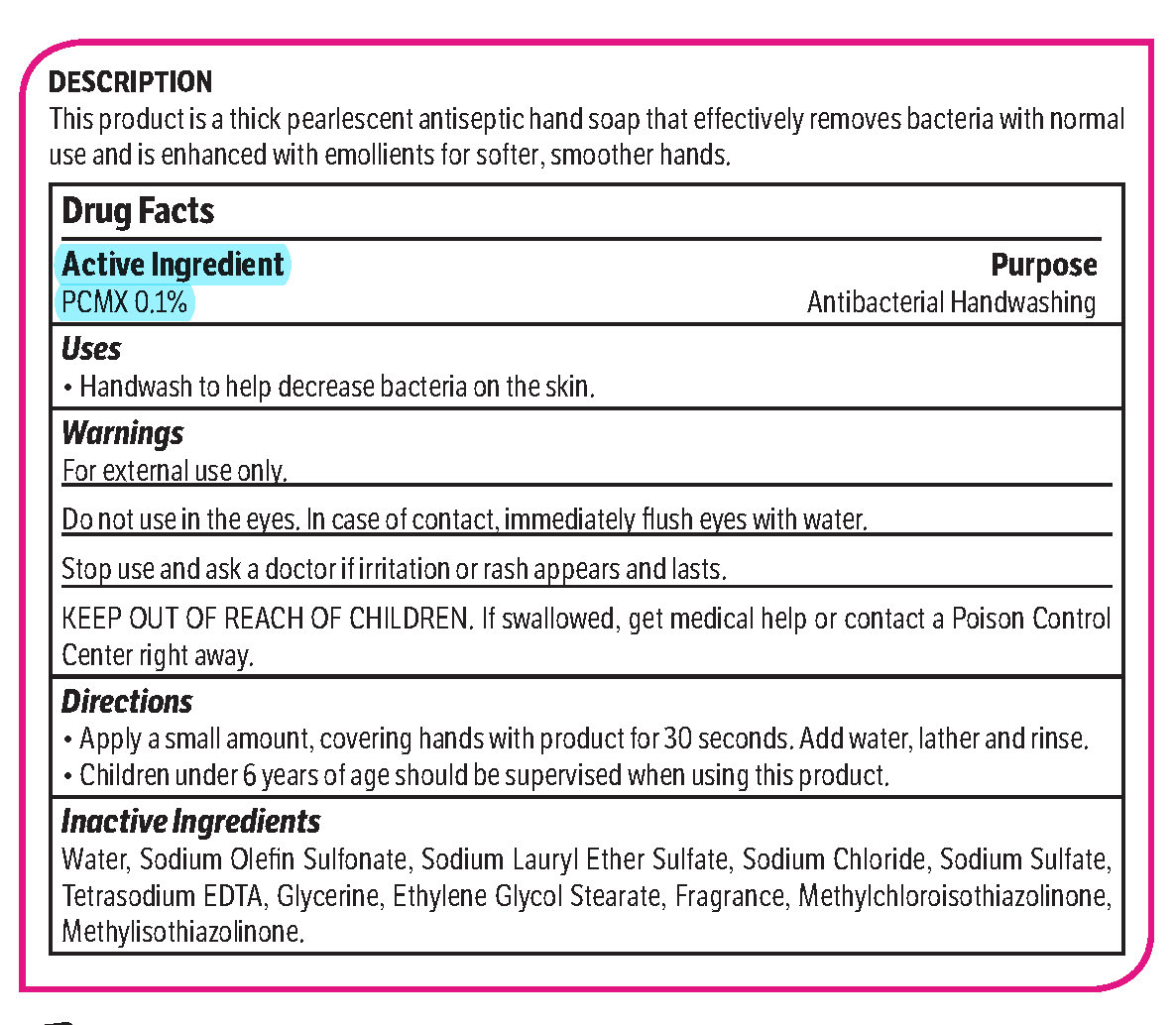
Bright Solutions antiseptic by is a Otc medication manufactured, distributed, or labeled by Midlab Incorporated. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BRIGHT SOLUTIONS ANTISEPTIC
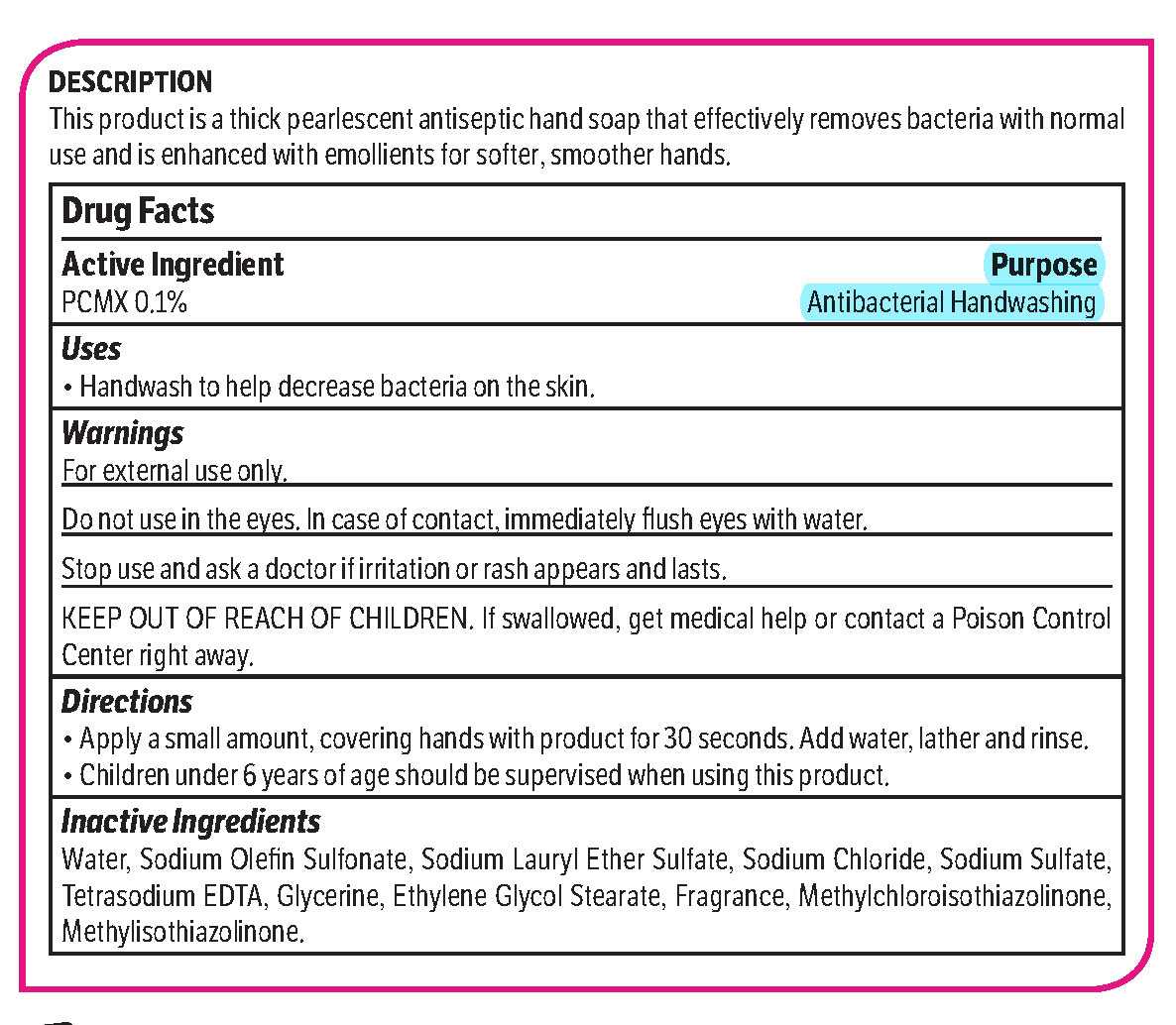
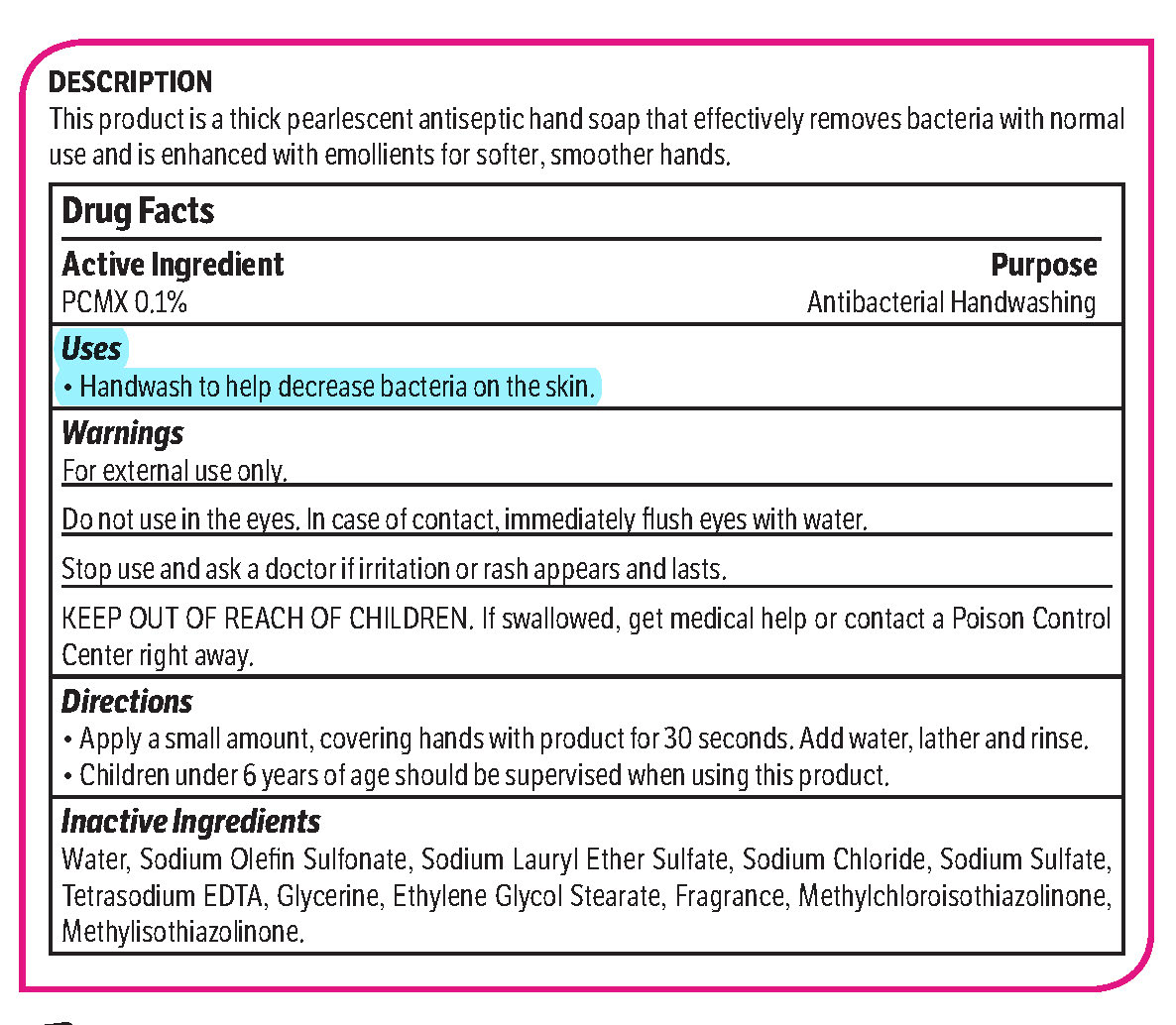
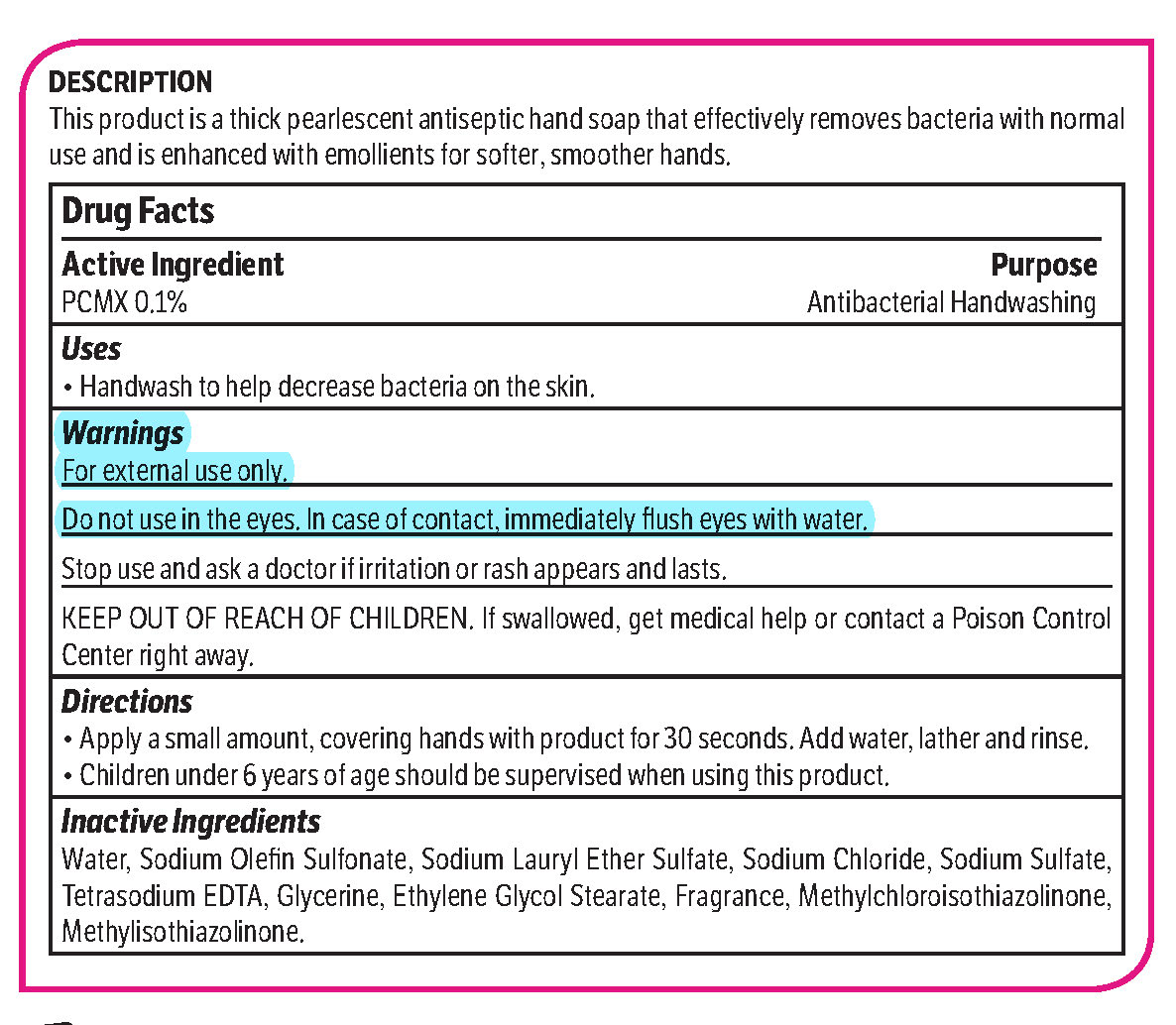
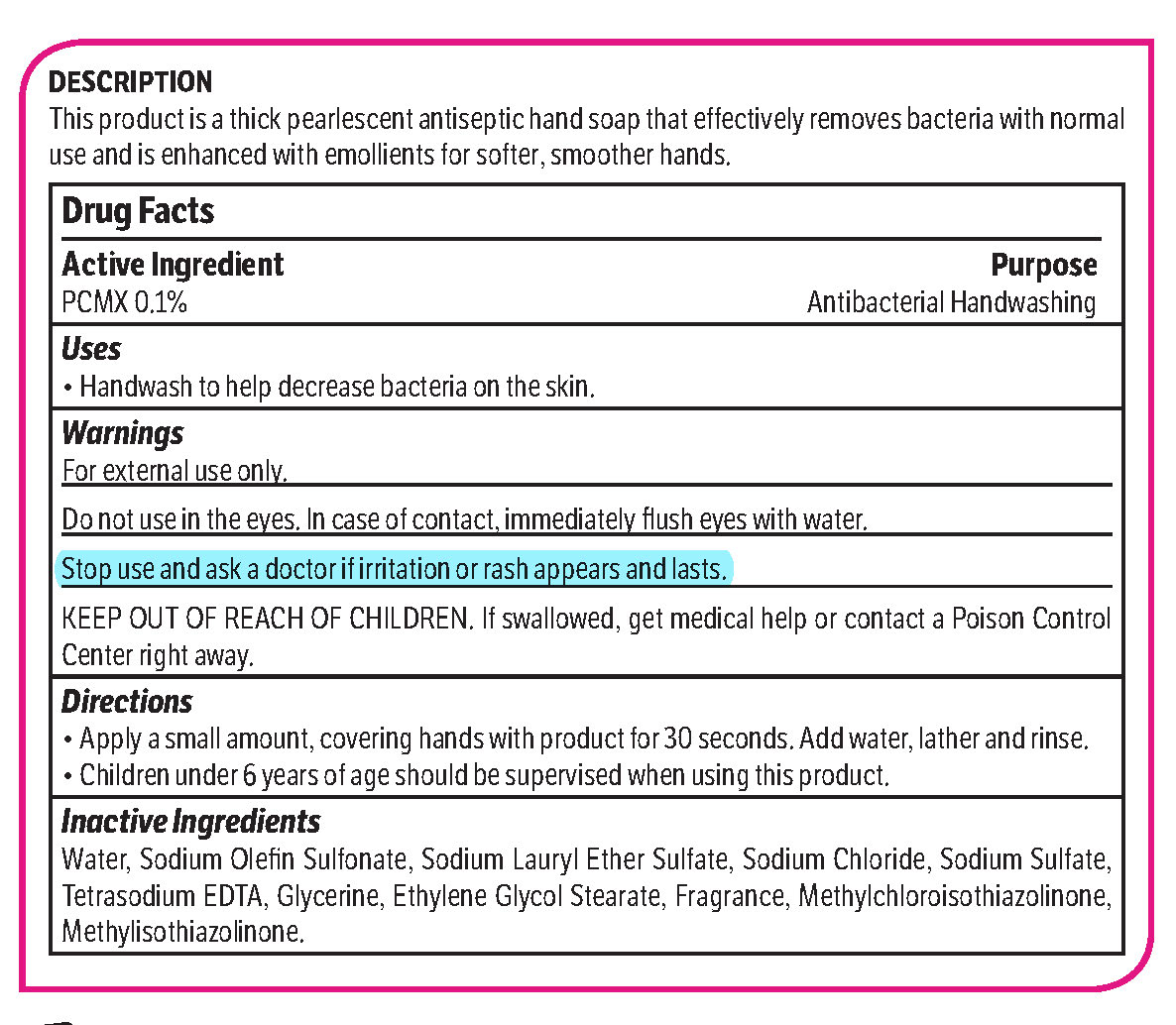
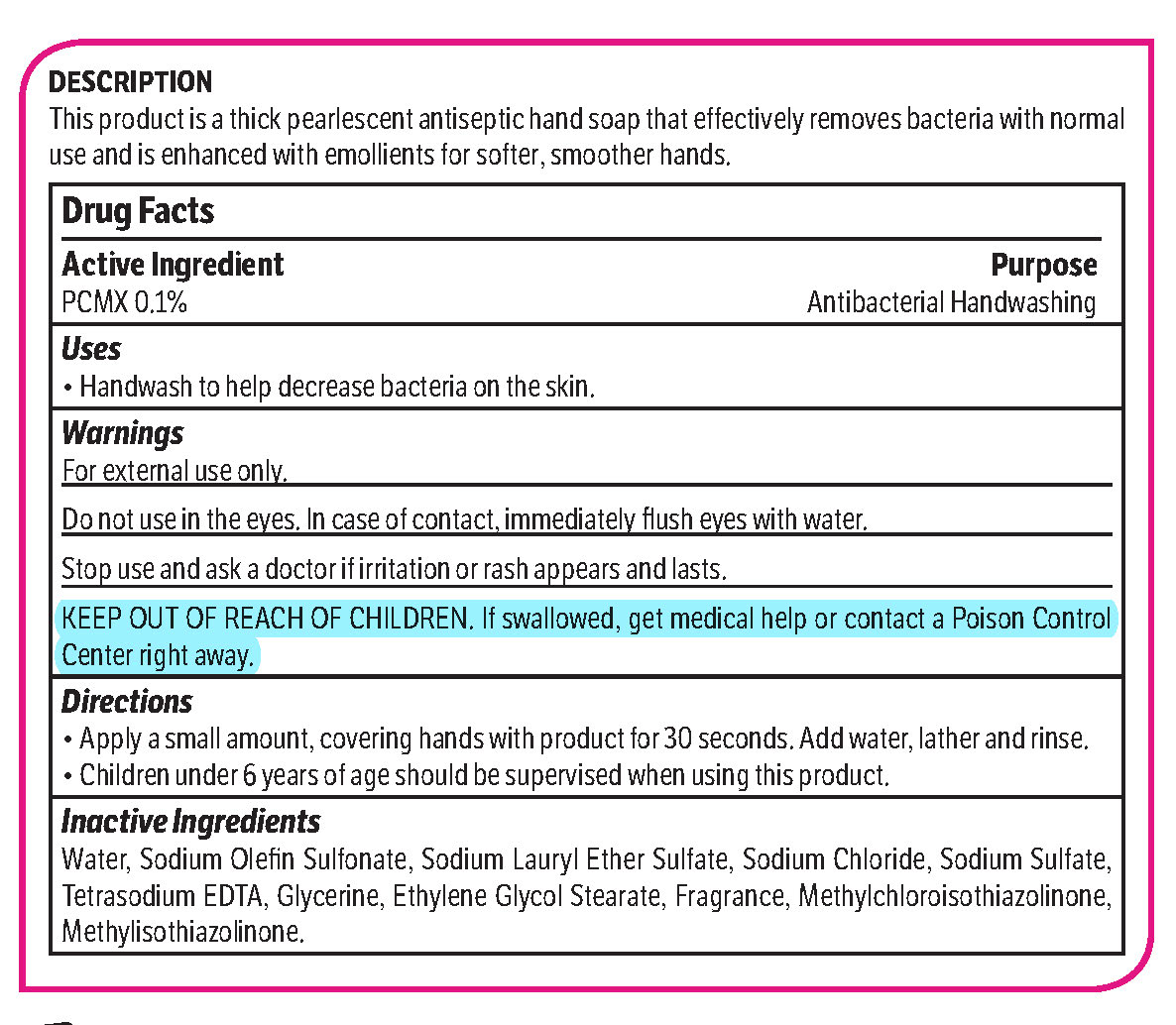
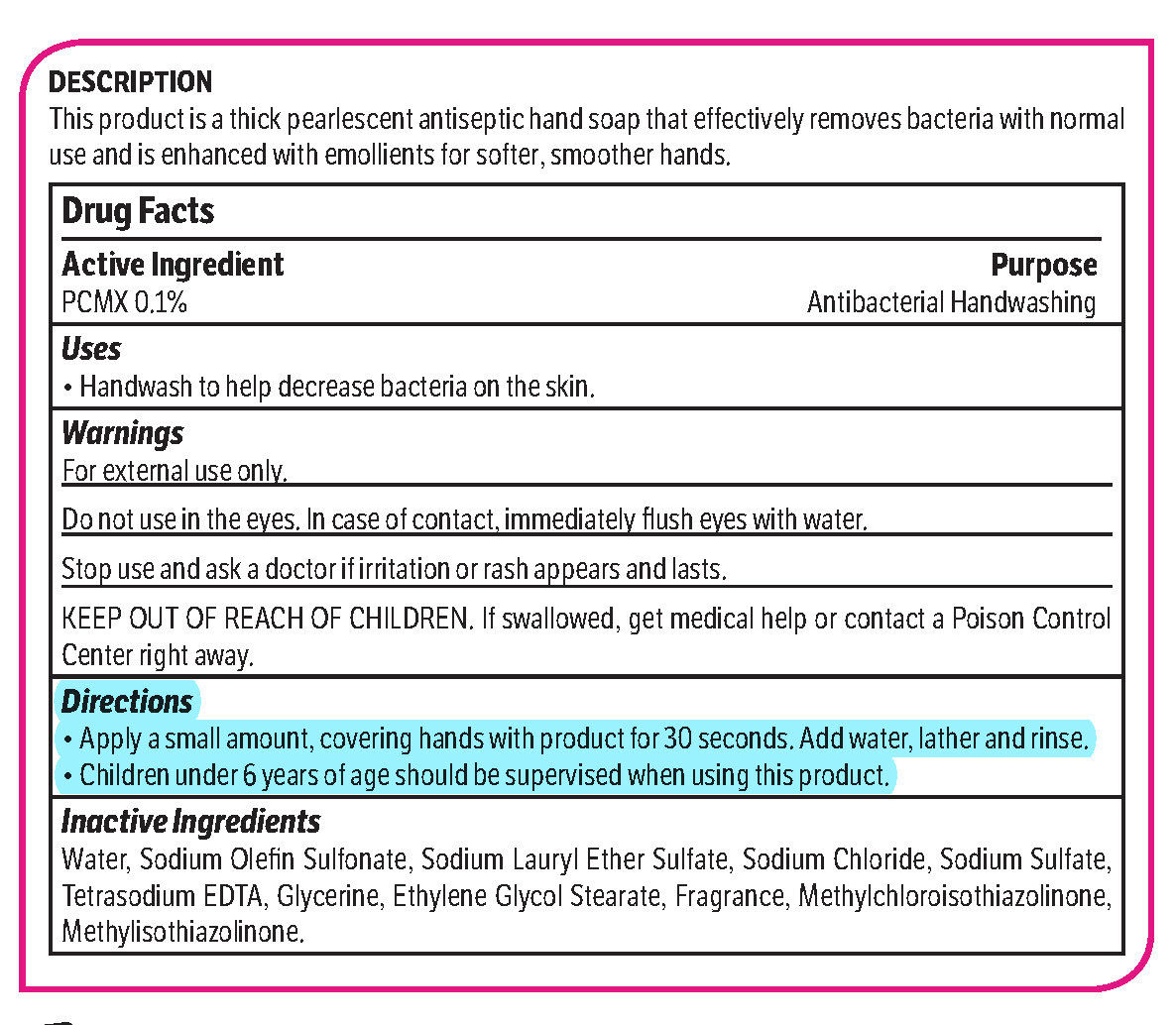
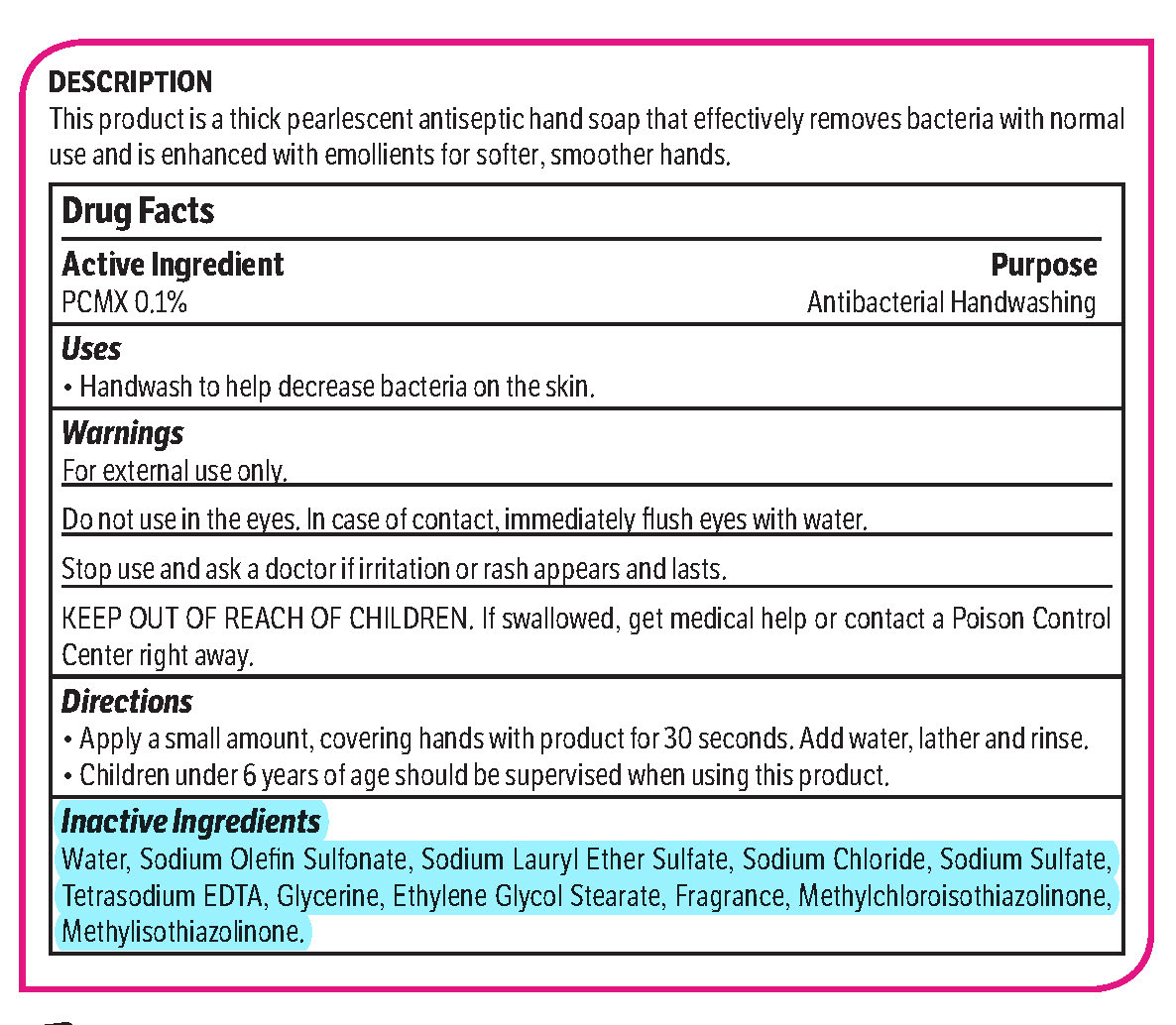
pcmx, hand soap soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 70542-303 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLOROXYLENOL (UNII: 0F32U78V2Q) (CHLOROXYLENOL - UNII:0F32U78V2Q) CHLOROXYLENOL 0.1 g in 1 L Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) GLYCOL STEARATE (UNII: 0324G66D0E) METHYLCHLOROISOTHIAZOLINONE/METHYLISOTHIAZOLINONE MIXTURE (UNII: 15O9QS218W) SODIUM SULFATE (UNII: 0YPR65R21J) TRIMETHYLENEDIAMINETETRAACETIC ACID (UNII: 3F6OA94EER) SODIUM C14-16 OLEFIN SULFONATE (UNII: O9W3D3YF5U) SODIUM LAURETH SULFATE (UNII: BPV390UAP0) GLYCERIN (UNII: PDC6A3C0OX) Product Characteristics Color white (Melon odor) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70542-303-41 3.78 L in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/01/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 02/01/2017 Labeler - Midlab Incorporated (047371463) Registrant - Midlab Incorporated (047371463) Establishment Name Address ID/FEI Business Operations Midlab Incorporated 047371463 manufacture(70542-303)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.