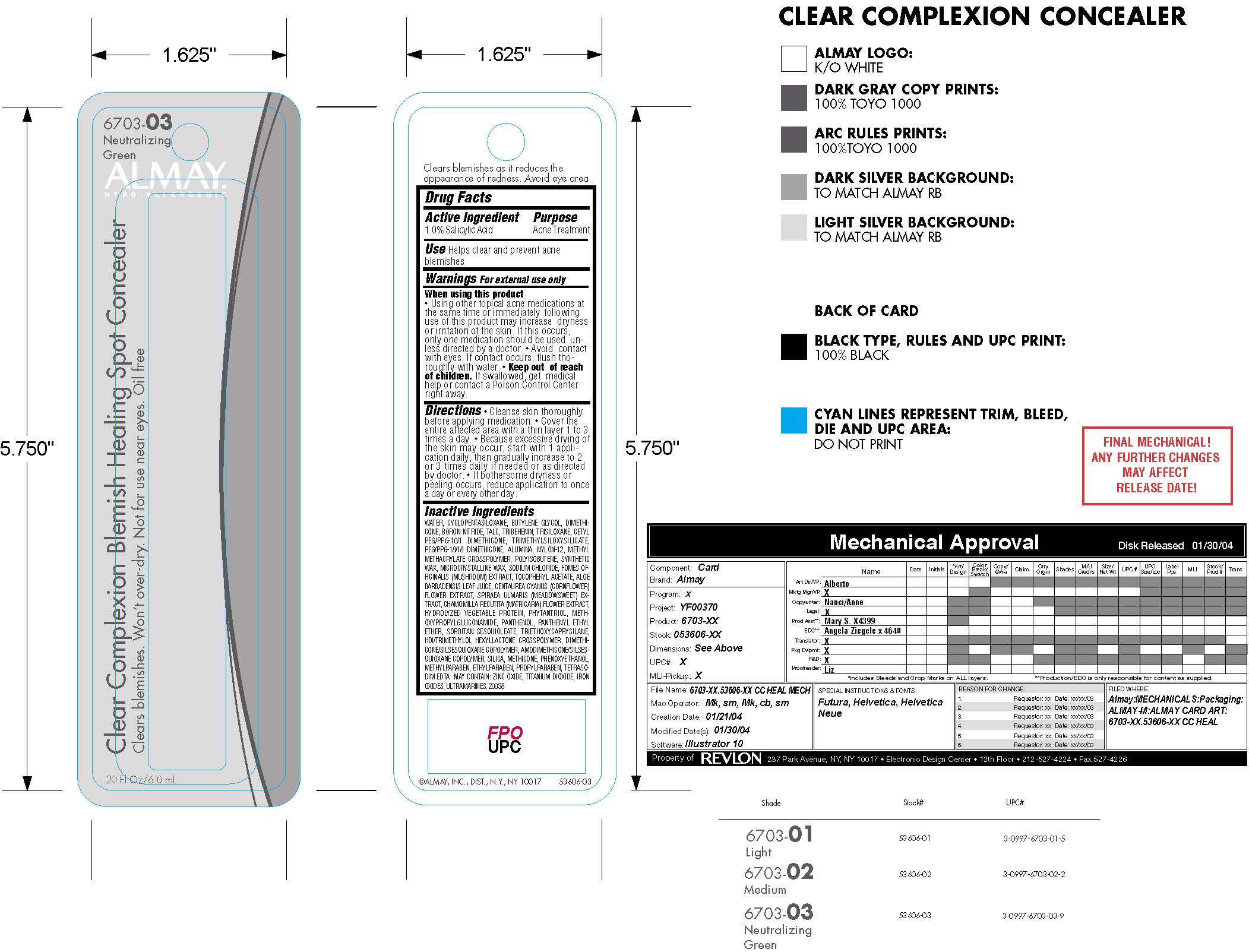
ALMAY CLEAR COMPLEXION BLEMISH HEALING SPOT CONCEALER- salicylic acid liquid
Almay Clear Complexion Blemish Healing Spot Concealer by
Drug Labeling and Warnings
Almay Clear Complexion Blemish Healing Spot Concealer by is a Otc medication manufactured, distributed, or labeled by Almay, Inc., REVLON, INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredients:
- Purpose
- Uses:
-
Warnings:
Using other topical acne medications at
the same time or immediately following
use of this product may increase dryness
or irritation of the skin. If this occurs,
only one medication should be used un -
less directed by a doctor. Avoid con tact
with eyes. If contact occurs, flush tho -
roughly with water. -
Directions
Cleanse skin thoroughly
before applying medication. Cover the
entire affected area with a thin layer 1 to 3
times a day. Because excessive drying of
the skin may occur, start with 1 appli -
cation daily, then gradually increase to 2
or 3 times daily if needed or as directed
by doctor. If bothersome dryness or
peeling occurs, reduce application to once
a day or every other day. -
Inactive Ingredients
WATER, CYCLOPENTASILOXANE, BUTYLENE GLYCOL, DIMETHICONE,
BORON NITRIDE, TALC, TRIBEHENIN, TRISILOXANE, CETYL
PEG/PPG-10/1 DIMETHICONE, TRIMETHYL SIL OXY SILI CATE,
PEG/PPG-18/18 DIMETHICONE, ALUMINA, NYLON-12, METHYL
METHACRYLATE CROSSPOLYMER, POLYISO BU TENE, SYNTHETIC
WAX, MICROCRYSTALLINE WAX, SODIUM CHLORIDE, FOMES OFFICINALIS
(MUSHROOM) EXTRACT, TOCOPHERYL ACETATE, ALOE
BARBADENSIS LEAF JUICE, CENTAUREA CYANUS (CORNFLOWER)
FLOWER EXTRACT, SPI RAEA ULMARIS (MEADOWSWEET) EXTRACT,
CHAMOMILLA RECUTITA (MATRICARIA) FLOWER EXTRACT,
HYDROLYZED VEGE TABLE PROTEIN, PHYTANTRIOL, METH -
OXYPROPYLGLUCONAMIDE, PANTHENOL, PANTHENYL ETHYL
ETHER, SORBITAN SESQUIOLEATE, TRIETHOXYCAPRYSILANE,
HDI/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER, DIMETHICONE/
SILSES QUI OXANE COPOLYMER, AMODIMETHICONE/SILSESQUIOXANE
COPOLYMER, SILICA, METHICONE, PHENOXYETHANOL,
METHYLPARABEN, ETHYLPARABEN, PROPYLPARABEN, TETRASODIM
EDTA MAY CONTAIN: ZINC OXIDE, TITANIUM DIOXIDE, IRON
OX IDES, ULTRAMARINES. 20038N - DOSAGE & ADMINISTRATION
- Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
ALMAY CLEAR COMPLEXION BLEMISH HEALING SPOT CONCEALER
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0311-0718 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 0.01 g in 1 mL Inactive Ingredients Ingredient Name Strength SYNTHETIC WAX (1200 MW) (UNII: Q3Z4BCH099) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) CULTIVATED MUSHROOM (UNII: 54C8E6W6JY) FILIPENDULA ULMARIA LEAF (UNII: 37GVN739ZQ) METHOXYPROPYLGLUCONAMIDE (UNII: GON1S528TS) PANTHENOL (UNII: WV9CM0O67Z) PANTHENYL ETHYL ETHER (UNII: F4WMF8NX3B) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) NYLON-12 (UNII: 446U8J075B) DIMETHICONE (UNII: 92RU3N3Y1O) BORON NITRIDE (UNII: 2U4T60A6YD) TRIMETHYLSILOXYSILICATE (M/Q 0.8-1.0) (UNII: 25LXE464L2) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TALC (UNII: 7SEV7J4R1U) SODIUM CHLORIDE (UNII: 451W47IQ8X) ALOE VERA LEAF (UNII: ZY81Z83H0X) CENTAUREA CYANUS FLOWER (UNII: QZ239038YC) TRISILOXANE (UNII: 9G1ZW13R0G) PHYTANTRIOL (UNII: 8LVI07A72W) TRIBEHENIN (UNII: 8OC9U7TQZ0) ALUMINUM OXIDE (UNII: LMI26O6933) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 1.5) (UNII: V2W71V8T0X) METHICONE (20 CST) (UNII: 6777U11MKT) EDETATE SODIUM (UNII: MP1J8420LU) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYLPARABEN (UNII: A2I8C7HI9T) ETHYLPARABEN (UNII: 14255EXE39) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0311-0718-20 6 mL in 1 TUBE; Type 0: Not a Combination Product 09/04/2007 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part358H 09/04/2007 Labeler - Almay, Inc. (064988652) Establishment Name Address ID/FEI Business Operations REVLON, INC. 809725570 manufacture(0311-0718)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.