OMVOH- mirikizumab-mrkz injection, solution OMVOH- mirikizumab-mrkz kit
Omvoh by
Drug Labeling and Warnings
Omvoh by is a Prescription medication manufactured, distributed, or labeled by Eli Lilly and Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use OMVOH safely and effectively. See full prescribing information for OMVOH.
OMVOH (mirikizumab-mrkz) injection, for intravenous or subcutaneous use
Initial U.S. Approval: 2023RECENT MAJOR CHANGES
Indications and Usage (1) 1/2025 Dosage and Administration Recommended Dosage for Ulcerative Colitis (2.2) 10/2025 Recommended Dosage for Crohn's Disease (2.3) 1/2025 Preparation and Administration Instructions for Intravenous Infusion (2.4) 1/2025 Preparation and Administration Instructions for Subcutaneous Injection(2.5) 10/2025 INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
Prior to Treatment Initiation
- Evaluate patients for tuberculosis (TB) infection. (2.1, 5.3)
- Obtain liver enzymes and bilirubin levels. (2.1, 5.4)
- Complete all age-appropriate vaccinations according to current immunization guidelines. (2.1, 5.5)
Recommended Dosage for Ulcerative Colitis
- Induction Dosage: Week 0, Week 4, and Week 8: Infuse 300 mg intravenously over at least 30 minutes. (2.2)
- Maintenance Dosage: Week 12 and every 4 weeks thereafter: Inject 200 mg subcutaneously (given as either one injection of 200 mg or as two consecutive injections of 100 mg each). (2.2)
Recommended Dosage for Crohn's Disease
- Induction Dosage: Week 0, Week 4, and Week 8: Infuse 900 mg intravenously over at least 90 minutes. (2.3)
- Maintenance Dosage: Week 12 and every 4 weeks thereafter: Inject 300 mg subcutaneously (given as two consecutive injections of 100 mg and 200 mg in any order). (2.3)
Preparation and Administration Instructions
DOSAGE FORMS AND STRENGTHS
Intravenous Infusion (3)
- Injection: 300 mg/15 mL (20 mg/mL) solution in a single-dose vial
Subcutaneous Injection (3):
- Injection: 100 mg/mL solution in a single-dose prefilled pen
- Injection: 100 mg/mL solution in a single-dose prefilled syringe
- Injection: 200 mg/2 mL solution in a single-dose prefilled pen
- Injection: 200 mg/2 mL (100 mg/mL) solution in a single-dose prefilled syringe
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Hypersensitivity Reactions: Serious hypersensitivity reactions, including anaphylaxis and infusion-related reactions, have been reported. If a severe hypersensitivity reaction occurs, discontinue and initiate appropriate treatment. (5.1)
- Infections: OMVOH may increase the risk of infection. Do not initiate treatment with OMVOH in patients with a clinically important active infection until the infection resolves or is adequately treated. If a serious infection develops, do not administer OMVOH until the infection resolves. (5.2)
- Tuberculosis: Do not administer OMVOH to patients with active TB infection. Monitor patients receiving OMVOH for signs and symptoms of active TB during and after treatment. (5.3)
- Hepatotoxicity: Drug-induced liver injury has been reported. Monitor liver enzymes and bilirubin levels at baseline and for at least 24 weeks of treatment and thereafter according to routine patient management. Interrupt treatment if drug-induced liver injury is suspected, until this diagnosis is excluded. (5.4)
- Immunizations: Avoid use of live vaccines. (5.5)
ADVERSE REACTIONS
Most common adverse reactions are:
- Ulcerative colitis (≥2%):
- Crohn's disease (≥5%): upper respiratory tract infections, injection site reactions, headache, arthralgia, and elevated liver tests (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Eli Lilly and Company at 1-800-LillyRx (1-800-545-5979) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 11/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Evaluations and Immunizations Prior to Treatment Initiation
2.2 Recommended Dosage for Ulcerative Colitis
2.3 Recommended Dosage for Crohn's Disease
2.4 Preparation and Administration Instructions for Intravenous Infusion
2.5 Preparation and Administration Instructions for Subcutaneous Injection
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Infections
5.3 Tuberculosis
5.4 Hepatotoxicity
5.5 Immunizations
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 CYP450 Substrates
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Ulcerative Colitis
14.2 Crohn's Disease
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Evaluations and Immunizations Prior to Treatment Initiation
- Evaluate patients for tuberculosis (TB) infection prior to initiating treatment with OMVOH [see Warnings and Precautions (5.3)].
- Obtain liver enzymes and bilirubin levels prior to initiating treatment with OMVOH [see Warnings and Precautions (5.4)].
- Complete all age-appropriate vaccinations according to current immunization guidelines [see Warnings and Precautions (5.5)].
2.2 Recommended Dosage for Ulcerative Colitis
Induction Dosage
Week 0, Week 4, and Week 8: Infuse 300 mg intravenously over at least 30 minutes [see Dosage and Administration (2.4)].
2.3 Recommended Dosage for Crohn's Disease
Induction Dosage
Week 0, Week 4, and Week 8: Infuse 900 mg intravenously over at least 90 minutes [see Dosage and Administration (2.4)].
2.4 Preparation and Administration Instructions for Intravenous Infusion
- OMVOH for intravenous use is intended for administration by a healthcare provider using aseptic technique.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The solution should be a clear to opalescent, colorless to slightly yellow to slightly brown solution, and free of visible particles. Do not use OMVOH if it is cloudy or there are visible particles.
-
Prior to intravenous administration, determine the dose needed based on the patient's indication (see Table 1 below). For Crohn's disease, discard 45 mL of the infusion bag prior to adding vial contents. Withdraw the required amount of solution from the vial(s) using a 18 gauge to 21 gauge needle and transfer to an infusion bag containing 0.9% Sodium Chloride Injection or 5% Dextrose Injection (see Table 1 below). Do not mix with other drugs. Do not dilute or infuse through the same intravenous line with other solutions.
Table 1: Intravenous Induction Dose and Volume of Diluent Required Indication Intravenous Induction Dose Number of OMVOH 300mg/15mL vials needed Volume of
0.9% Sodium Chloride or
5% Dextrose InjectionUlcerative colitis 300 mg 1 50 mL, 100 mL, or 250 mL Crohn's disease 900 mg 3 100 mL or 250 mL - Gently invert the infusion bag to mix the contents. Do not shake the prepared infusion bag.
- Connect the intravenous administration set (infusion line) to the prepared infusion bag and prime the line.
- Infuse the diluted solution intravenously over a period of at least 30 minutes for the 300 mg dose; at least 90 minutes for the 900 mg dose. If stored refrigerated, allow the diluted solution in the infusion bag to warm to room temperature prior to the start of the intravenous infusion.
- At the end of the infusion, flush the line with 0.9% Sodium Chloride Injection or 5% Dextrose Injection.
- Administer the flush at the same infusion rate as used for OMVOH administration.
- The time required to flush OMVOH solution from the infusion line is in addition to the minimum 30-minute infusion time.
Storage of Diluted Solution
- Start the infusion immediately after preparation. If not used immediately, store the diluted infusion solution in the refrigerator at 2°C to 8°C (36°F to 46°F). Use the diluted infusion solution within 48 total hours, of which not more than 5 hours are permitted at non-refrigerated temperatures not to exceed 25°C (77°F), starting from the time of vial puncture.
- Keep drug product away from direct heat or light. Do not freeze the diluted solution in the prepared infusion bag.
2.5 Preparation and Administration Instructions for Subcutaneous Injection
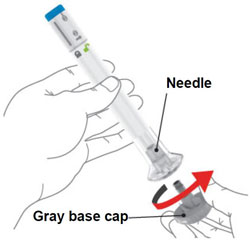
- OMVOH is intended for use under the guidance and supervision of a healthcare professional. Patients may self-inject OMVOH after training in subcutaneous injection technique. Provide proper training to patients and/or caregivers on the subcutaneous injection technique of OMVOH according to the “Instructions for Use”, included with the packaged product.
- Before injection, remove OMVOH prefilled pens or OMVOH prefilled syringes from the refrigerator, and leave at room temperature for 45 minutes.
- Do not shake the prefilled pens or prefilled syringes.
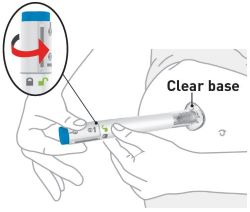
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The solution should be a clear to opalescent, colorless to slightly yellow to slightly brown solution, and free of visible particles. Do not use OMVOH if it is cloudy, discolored, or there are visible particles.
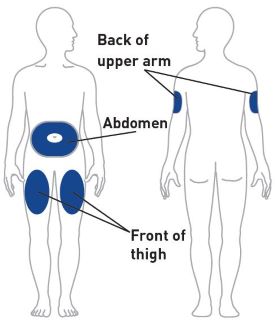
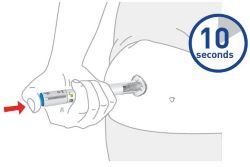
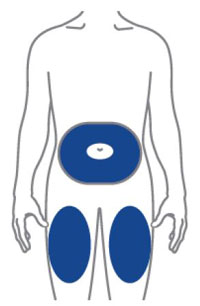
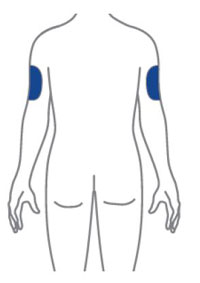
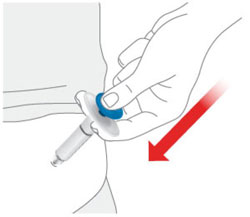
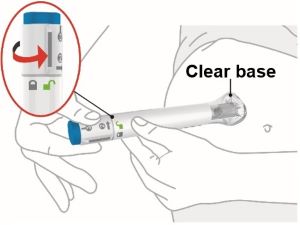
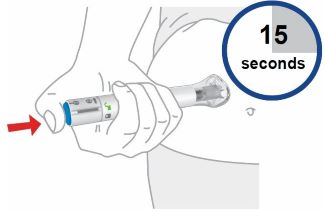
- Sites for injection include the abdomen, thigh, and back of the upper arm. Instruct patients to inject in a different location (such as upper arms, thighs or any quadrant of abdomen) than the previous injection. Administration of OMVOH in the back of upper arm may only be performed by another person.
- Do not inject into areas where the skin is tender, bruised, erythematous, or indurated.
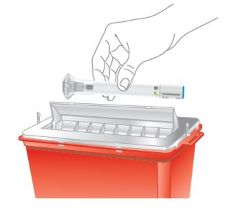
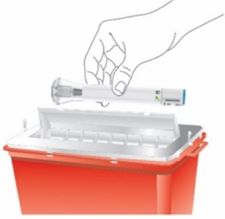
- OMVOH does not contain preservatives; therefore, discard any unused product. Do not reuse.
- If a dose is missed, administer the dose as soon as possible. Thereafter, resume dosing every 4 weeks.
-
3 DOSAGE FORMS AND STRENGTHS
OMVOH is a clear to opalescent, colorless to slightly yellow to slightly brown solution available as:
- Intravenous Infusion:
Injection: 300 mg/15 mL (20 mg/mL) solution in a single-dose vial - Subcutaneous Use:
Injection: 100 mg/mL solution in a single-dose prefilled pen
Injection: 100 mg/mL solution in a single-dose prefilled syringe
Injection: 200 mg/2 mL solution in a single-dose prefilled pen
Injection: 200 mg/2 mL (100 mg/mL) solution in a single-dose prefilled syringe
- Intravenous Infusion:
-
4 CONTRAINDICATIONS
OMVOH is contraindicated in patients with a history of serious hypersensitivity reaction to mirikizumab-mrkz or any of the excipients [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Serious hypersensitivity reactions, including anaphylaxis during intravenous infusion, have been reported with OMVOH administration. Infusion-related hypersensitivity reactions, including mucocutaneous erythema and pruritus, were reported during induction [see Adverse Reactions (6.1)]. If a severe hypersensitivity reaction occurs, discontinue OMVOH immediately and initiate appropriate treatment.
5.2 Infections
OMVOH may increase the risk of infection [see Adverse Reactions (6.1)].
Do not initiate treatment with OMVOH in patients with a clinically important active infection until the infection resolves or is adequately treated.
In patients with a chronic infection or a history of recurrent infection, consider the risks and benefits prior to prescribing OMVOH. Instruct patients to seek medical advice if signs or symptoms of clinically important acute or chronic infection occur. If a serious infection develops or an infection is not responding to standard therapy, monitor the patient closely and do not administer OMVOH until the infection resolves.
5.3 Tuberculosis
Evaluate patients for tuberculosis (TB) infection prior to initiating treatment with OMVOH.
Do not administer OMVOH to patients with active TB infection. Initiate treatment of latent TB prior to administering OMVOH. Consider anti-TB therapy prior to initiation of OMVOH in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed. Monitor patients for signs and symptoms of active TB during and after OMVOH treatment.
In clinical trials, subjects were excluded if they had evidence of active TB, a past history of active TB, or were diagnosed with latent TB at screening.
5.4 Hepatotoxicity
A case of drug-induced liver injury (alanine aminotransferase [ALT] 18x the upper limit of normal (ULN), aspartate aminotransferase [AST] 10x ULN, and total bilirubin 2.4x ULN) in conjunction with pruritus was reported in a clinical trial subject following a longer than recommended induction regimen. OMVOH was discontinued. Liver test abnormalities eventually returned to baseline.
Evaluate liver enzymes and bilirubin at baseline and for at least 24 weeks of treatment. Monitor thereafter according to routine patient management.
Consider other treatment options in patients with evidence of liver cirrhosis. Prompt investigation of the cause of liver enzyme elevation is recommended to identify potential cases of drug-induced liver injury. Interrupt treatment if drug-induced liver injury is suspected, until this diagnosis is excluded. Instruct patients to seek immediate medical attention if they experience symptoms suggestive of hepatic dysfunction.
5.5 Immunizations
Avoid use of live vaccines in patients treated with OMVOH. Medications that interact with the immune system may increase the risk of infection following administration of live vaccines. Prior to initiating therapy with OMVOH, complete all age-appropriate vaccinations according to current immunization guidelines. No data are available on the response to live or non-live vaccines in patients treated with OMVOH.
-
6 ADVERSE REACTIONS
The following topics are also discussed in detail in the Warnings and Precautions section:
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
- Infections [see Warnings and Precautions (5.2)]
- Tuberculosis [see Warnings and Precautions (5.3)]
- Hepatotoxicity [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Ulcerative Colitis
OMVOH was studied up to 12 weeks in subjects with moderately to severely active ulcerative colitis in a randomized, double-blind, placebo-controlled induction study (UC-1). In subjects who responded to induction therapy in UC-1, long term safety up to 52 weeks was evaluated in a randomized, double-blind, placebo-controlled maintenance study (UC-2) and a long-term extension study [see Clinical Studies (14.1)].
In the induction study (UC-1), 1279 subjects were enrolled of whom 958 received OMVOH 300 mg administered as an intravenous infusion at Weeks 0, 4, and 8. In the maintenance study (UC-2), 581 subjects were enrolled of whom 389 received OMVOH 200 mg administered as a subcutaneous injection every 4 weeks.
Table 2 summarizes the adverse reactions reported in at least 2% of subjects and at a higher frequency than placebo during UC-1.
Table 2: Adverse Reactionsa in Subjects with Ulcerative Colitis through Week 12 in a Placebo-Controlled Induction Study (UC-1) a Reported in at least 2% of subjects and at a higher frequency than placebo.
b OMVOH 300 mg as an intravenous infusion at Weeks 0, 4, and 8.
c Upper respiratory tract infections includes related terms (e.g., COVID-19, nasopharyngitis, pharyngitis, rhinitis, sinusitis, and upper respiratory tract infection).
Adverse Reactions OMVOH
300 mg Intravenous Infusionb
N=958
n (%)Placebo
N=321
n (%)Upper respiratory tract infectionsc 72 (8%) 20 (6%) Arthralgia 20 (2%) 4 (1%) In the induction study (UC-1), infusion-related hypersensitivity reactions were reported by 4 (0.4%) subjects treated with OMVOH and 1 (0.3%) subject treated with placebo.
Table 3 summarizes the adverse reactions reported in at least 2% of subjects and at a higher frequency than placebo during the 40-week controlled period of UC-2.
Table 3: Adverse Reactionsa in Subjects with Ulcerative Colitis through Week 40 In a Placebo-Controlled Maintenance Study (UC-2) a Reported in at least 2% of subjects and at a higher frequency than placebo
b OMVOH 200 mg as a subcutaneous injection at Week 12 and every 4 weeks thereafter for up to an additional 40 weeks.
c Upper respiratory tract infections includes related terms (e.g., COVID-19, nasopharyngitis, pharyngitis, rhinitis, sinusitis, and upper respiratory tract infection).
d Injection site reactions includes related terms (e.g., erythema, hypersensitivity, pain, reaction, and urticaria at the injection site).
e Rash is composed of several similar terms.
f Herpes viral infection includes related terms (e.g., herpes zoster, herpes simplex, and oral herpes).
Adverse Reactions OMVOH
200 mg Subcutaneous Injectionb
N=389
n (%)Placebo
N=192
n (%)Upper respiratory tract infectionsc 53 (14%) 23 (12%) Injection site reactionsd 34 (9%) 8 (4%) Arthralgia 26 (7%) 8 (4%) Rashe 16 (4%) 2 (1%) Headache 16 (4%) 2 (1%) Herpes viral infectionf 9 (2%) 1 (1%) Infections
In UC-1 through Week 12, infections were reported by 145 (15%) subjects treated with OMVOH 300 mg and 45 (14%) subjects treated with placebo. Serious infections were reported by less than 1% in both groups. Serious infections in the OMVOH group included intestinal sepsis, listeria sepsis, and pneumonia.
In the maintenance study (UC-2) through Week 40 (a total of 52 weeks of treatment), infections were reported by 93 (24%) subjects treated with OMVOH 200 mg and 44 (23%) subjects treated with placebo. A case of COVID-19 pneumonia was reported as a serious infection in the OMVOH group.
Hepatic Enzyme Elevations
In UC-1 through Week 12, alanine aminotransferase (ALT) ≥5X ULN was reported by 1 (0.1%) subject treated with OMVOH 300 mg and 1 (0.3%) subject treated with placebo. Aspartate aminotransferase (AST) ≥5X ULN was reported by 2 (0.2%) subjects treated with OMVOH 300 mg and no subject treated with placebo. These elevations have been noted with and without concomitant elevations in total bilirubin.
In UC-2 through Week 40 (a total of 52 weeks of treatment), 3 (0.8%) subjects treated with OMVOH 200 mg reported ALT ≥5X ULN and 3 (0.8%) subjects reported AST ≥5X ULN; with or without concomitant elevations in total bilirubin. No subjects treated with placebo experienced similar elevations [see Warnings and Precautions (5.4)].
Crohn's Disease
OMVOH was studied up to 52 weeks in subjects with moderately to severely active Crohn's disease in a randomized, double-blind, placebo-controlled study (CD-1). The safety population consisted of 630 subjects who received OMVOH 900 mg administered as an intravenous infusion during induction at Weeks 0, 4, and 8 followed by OMVOH 300 mg administered as a subcutaneous injection every 4 weeks and 211 subjects who received placebo [see Clinical Trials (14.2)]. Eighty-five of the 211 placebo subjects in the safety population who did not achieve clinical response by patient-reported outcome at Week 12 were switched to blinded induction and maintenance treatment with OMVOH. Observed data from these 85 subjects are included in the placebo cohort up to Week 12 and in the OMVOH cohort after Week 12. In CD-1, OMVOH and placebo-treated subjects had different lengths of exposure, therefore, exposure adjusted incidence rates (EAIRs) are also displayed in Table 4 to compare adverse reactions.
Common Adverse Reactions
Table 4 summarizes the frequencies and EAIRs per 100 person-years (PY) for adverse reactions reported in at least 5% of subjects treated with OMVOH and at a higher frequency than placebo during CD-1.
Table 4: Adverse Reactionsa in Subjects with Crohn's Disease through Week 52 In a Placebo-Controlled Study (CD-1) Abbreviations: EAIR, exposure-adjusted incidence rate; PY, person-years
a Reported in at least 5% of subjects and at a higher frequency than placebo
b Following OMVOH 900 mg as an intravenous infusion at Week 0, Week 4, and Week 8, subjects received OMVOH 300 mg as a subcutaneous injection at Week 12 and every 4 weeks thereafter for up to an additional 40 weeks. In addition, eighty-five placebo subjects who did not achieve clinical response by patient-reported outcome at Week 12 were switched to blinded induction and maintenance treatment with OMVOH. The observed data after Week 12 from these eighty-five subjects were included in the OMVOH cohort.
c EAIRs are per 100 PY. The EAIR per 100 PY can be interpreted as an estimated number of first occurrences of the adverse reaction of interest if 100 subjects were treated for one year.
d Upper respiratory tract infections includes related terms (e.g., COVID-19, nasopharyngitis, pharyngitis, rhinitis, sinusitis, and upper respiratory tract infection).
e Injection site reactions includes related terms (e.g., erythema, hematoma, induration, pain, pruritus, and reaction at the injection site).
f Headache includes related terms (i.e., headache and migraine).
g Elevated liver tests include related terms (e.g., ALT increased, AST increased, alkaline phosphatase increased, bilirubin increased, and GGT increased)
Adverse Reactions OMVOHb
N=715, PY=655
n (%) [EAIRc]Placebo
N=211, PY=120
n (%) [EAIRc]Upper respiratory tract infectionsd 199 (28) [37] 47 (22) [47] Injection site reactionse 69 (10) [11] 8 (4) [7] Headachef 45 (6) [7] 9 (4) [8] Arthralgia 44 (6) [7] 11 (5) [10] Elevated liver testsg 36 (5) [6] 8 (4) [7] Hepatic Enzyme Elevations
Of the subjects with reported adverse reactions of elevated liver tests (see Table 4), 3 subjects treated with OMVOH reported ALT ≥5X ULN and 2 subjects reported AST ≥5X ULN. Neither subject had a concomitant elevation in total bilirubin. No subjects treated with placebo experienced similar elevations.
Less Common Adverse Reactions (<5%)
In CD-1 through Week 52, urticaria was reported by 13 (2%, 2 per 100 PY) subjects treated with OMVOH and no subjects treated with placebo.
Infections
In CD-1 through Week 52, infections were reported by 282 (39%, 58 per 100 PY) subjects treated with OMVOH and 73 (35%, 81 per 100 PY) subjects treated with placebo. Serious infections in the OMVOH group included abscess (including abdominal abscess, anal abscess, gluteal abscess, and perineal abscess), cellulitis, pneumonia, and sepsis.
-
7 DRUG INTERACTIONS
7.1 CYP450 Substrates
Increased concentrations of cytokines (e.g., IL-1, IL-6, IL-10, TNFα, IFN) during chronic inflammation associated with certain diseases including Crohn's disease may suppress the formation of CYP450 enzymes. Therapeutic proteins, including mirikizumab-mrkz, that decrease the concentrations of these pro-inflammatory cytokines may increase the formation of CYP450 enzymes resulting in decreased CYP450 substrate exposure. Upon initiation or discontinuation of OMVOH in patients treated with concomitant CYP450 substrates, monitor drug concentrations or other therapeutic parameters, and adjust the dosage of the CYP450 substrate as needed. See the prescribing information of specific CYP450 substrates.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There will be a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to OMVOH during pregnancy. Pregnant women exposed to OMVOH and healthcare providers are encouraged to call Eli Lilly and Company at 1-800-Lilly-Rx (1-800-545-5979).
Risk Summary
Available data from case reports of mirikizumab-mrkz use in pregnant women are insufficient to evaluate for a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Although there are no data on mirikizumab-mrkz, monoclonal antibodies can be actively transported across the placenta, and mirikizumab-mrkz may cause immunosuppression in the in utero-exposed infant. An enhanced pre- and post-natal development study conducted in pregnant monkeys at a dose 20 times the maximum recommended human dose (MRHD) revealed no adverse developmental effects to the developing fetus, or harm to infant monkeys from birth through 6 months of age. There are risks of adverse pregnancy outcomes associated with increased disease activity in women with inflammatory bowel disease (see Clinical Considerations).
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and Embryo/Fetal Risk
Published data suggest that the risk of adverse pregnancy outcomes in women with inflammatory bowel disease (IBD) is associated with increased disease activity. Adverse pregnancy outcomes include preterm delivery (before 37 weeks gestation), low birth weight (less than 2500 g) infants, and small for gestational age at birth.
Fetal/Neonatal Adverse Reactions
Transport of endogenous IgG antibodies across the placenta increases as pregnancy progresses, and peaks during the third trimester. It is unclear whether mirikizumab-mrkz may interfere with an infant's immune response to infections. Therefore, monitoring for the development of serious infection during the first 2 months of life in infants exposed in utero is recommended.
Data
Animal Data
An enhanced pre- and postnatal development study was conducted in cynomolgus monkeys administered mirikizumab-mrkz by intravenous injection during organogenesis to parturition at a dose of 300 mg/kg twice weekly (20 times the MRHD based on exposure comparisons). Mirikizumab-mrkz crossed the placenta in monkeys. No maternal toxicity was noted in this study. No mirikizumab-mrkz-related effects on morphological, functional or immunological development were observed in infant monkeys from birth through 6 months of age. However, incidences of embryo/fetal loss were higher in the treated groups compared to control (6.7% [1 of 15] in controls vs 26.7% [4 of 15] at 300 mg/kg (20 times the MRHD, based on exposure comparisons) but were within the range of historical control data. Following delivery, most adult female cynomolgus monkeys and all infants from the mirikizumab-mrkz-treated group had measurable serum concentrations up to 28 days postpartum. In the infant monkeys, mean serum concentrations were approximately 4.8 times the respective mean maternal concentrations.
8.2 Lactation
Risk Summary
There are no data on the presence of mirikizumab-mrkz in human milk, the effects on the breastfed infant, or the effects on milk production. Endogenous maternal IgG and monoclonal antibodies are transferred in human milk. The effects of local gastrointestinal exposure and limited systemic exposure in the breastfed infant to mirikizumab-mrkz are unknown. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for OMVOH and any potential adverse effects on the breastfed infant from OMVOH or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of OMVOH have not been established in pediatric patients.
8.5 Geriatric Use
Of the 795 OMVOH-treated subjects in the two ulcerative colitis clinical studies, 64 subjects (8%) were 65 years of age and older, while 10 subjects (1%) were 75 years of age and older. Of the 715 OMVOH-treated subjects in the Crohn's disease clinical study, 22 subjects (3%) were 65 years of age and older, while 6 subjects (1%) were 75 years of age and older). These clinical studies did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger adult subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger subjects. No clinically meaningful differences in the pharmacokinetics of mirikizumab-mrkz were observed in subjects 65 years of age and older compared to younger adult subjects [see Clinical Pharmacology (12.3)].
-
11 DESCRIPTION
Mirikizumab-mrkz is a humanized immunoglobulin G4 (IgG4) variant monoclonal antibody that is directed against the p19 subunit of IL-23 and does not bind IL-12. Mirikizumab-mrkz is produced in Chinese Hamster Ovary (CHO) cells by recombinant DNA technology and it is composed of two identical light chain polypeptides and two identical heavy chain polypeptides with an overall molecular weight of approximately 147 kDa.
OMVOH for intravenous infusion for ulcerative colitis and Crohn's disease
OMVOH (mirikizumab-mrkz) injection is a sterile, preservative-free, clear to opalescent, colorless to slightly yellow to slightly brown solution in a single-dose vial for intravenous infusion after dilution.
OMVOH injection 300 mg /15 mL vial is available in 2 formulations:
- Each mL contains 20 mg of mirikizumab-mrkz, anhydrous citric acid (0.4 mg), polysorbate 80 (0.5 mg), sodium chloride (8.8 mg), sodium citrate (2.1 mg), and Water for Injection. The OMVOH solution has a pH range of 5.0 to 6.0.
- Each mL contains 20 mg of mirikizumab-mrkz, anhydrous citric acid (0.1 mg), histidine (0.1 mg), L-histidine hydrochloride monohydrate (0.9 mg), mannitol (33 mg), polysorbate 80 (0.5 mg), sodium chloride (2.9 mg), sodium citrate (0.2 mg), and Water for Injection. The OMVOH solution has a pH range of 5.0 to 5.8.
OMVOH (mirikizumab-mrkz) injection 100 mg/mL prefilled pen or prefilled syringe for subcutaneous use for ulcerative colitis and Crohn's disease
OMVOH (mirikizumab-mrkz) injection is a sterile, preservative free, clear to opalescent, colorless to slightly yellow to slightly brown solution for subcutaneous use available as 100 mg of mirikizumab-mrkz in a 1 mL single-dose prefilled pen or single-dose prefilled syringe. The prefilled pen and prefilled syringe contain a 1 mL glass syringe with a fixed 27-gauge ½ inch needle. The OMVOH 100 mg prefilled pen and prefilled syringe are manufactured to deliver 100 mg of mirikizumab-mrkz.
Each mL is composed of 100 mg mirikizumab-mrkz, histidine (0.1 mg), L-histidine hydrochloride monohydrate (0.9 mg), mannitol (33 mg), polysorbate 80 (0.3 mg), sodium chloride (2.9 mg), and Water for Injection. The OMVOH solution has a pH range of 5.0 to 5.8.
OMVOH (mirikizumab-mrkz) injection 200 mg/2 mL prefilled pen or 200 mg/2 mL (100 mg/mL) prefilled syringe for subcutaneous use for ulcerative colitis and Crohn's disease
OMVOH (mirikizumab-mrkz) injection is a sterile, preservative free, clear to opalescent, colorless to slightly yellow to slightly brown solution for subcutaneous use. The 2 mL prefilled pen and prefilled syringe each contain a 2 mL glass syringe with a fixed 27-gauge 8 mm needle. The OMVOH 200 mg prefilled pen and prefilled syringe are manufactured to deliver 200 mg of mirikizumab-mrkz.
Each mL is composed of 100 mg mirikizumab-mrkz, histidine (0.1 mg), L-histidine hydrochloride monohydrate (0.9 mg), mannitol (33 mg), polysorbate 80 (0.3 mg), sodium chloride (2.9 mg), and Water for Injection. The OMVOH solution has a pH range of 5.0 to 5.8.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Mirikizumab-mrkz is a humanized IgG4 monoclonal antibody that selectively binds to the p19 subunit of human IL-23 cytokine and inhibits its interaction with the IL-23 receptor.
IL-23 is involved in mucosal inflammation and affects the differentiation, expansion, and survival of T cell subsets, and innate immune cell subsets, which represent sources of pro-inflammatory cytokines. Research in animal models has shown that pharmacologic inhibition of IL-23p19 can ameliorate intestinal inflammation.
Mirikizumab-mrkz inhibits the release of pro-inflammatory cytokines and chemokines.
12.2 Pharmacodynamics
In both study UC-1 (induction) and study UC-2 (maintenance), a positive relationship was observed between Mirikizumab-mrkz average concentration and rates of clinical remission and clinical response [see Clinical Studies (14.1)].
Mirikizumab exposure response relationships have not been fully characterized in subjects with Crohn's disease.
12.3 Pharmacokinetics
Mirikizumab-mrkz exhibited linear pharmacokinetics with dose-proportional increase in exposure over a dose range of 60 to 2400 mg given as an intravenous injection or over a dose range of 200 to 400 mg given as a subcutaneous injection, in healthy volunteers. There was no apparent accumulation of mirikizumab-mrkz concentrations in serum over time when administered as a subcutaneous injection every 4 weeks to subjects with ulcerative colitis or Crohn's disease. The estimated exposure parameters of mirikizumab-mrkz at steady state are summarized in Tables 5 for ulcerative colitis and 6 for Crohn's disease.
Table 5: Mirikizumab-mrkz Estimated Systemic Exposure at Steady State in Subjects with Ulcerative Colitis a OMVOH 300 mg as an intravenous infusion over at least 30 minutes at Weeks 0, 4, and 8.
b OMVOH 200 mg as 2 subcutaneous injections (100 mg/mL each) at Week 12 and every 4 weeks thereafter for up to an additional 40 weeks.
c AUCtau, ss = area under the concentration-versus-time curve over one dosing interval at steady state; Cmax, ss = maximum concentration at steady state; Ctrough, ss = concentration at the end of the dosing interval at steady state; CV = geometric coefficient of variation.
OMVOH
300 mg Intravenous Infusiona
Geometric mean (CV%)OMVOH
200 mg Subcutaneous Injectionb
Geometric mean (CV%)Cmax, ss (μg/mL)c 99.7 (22.7%) 10.1 (52.1%) AUCtau, ss (μg*day/mL)c 538 (34.4%) 160 (57.6%) Ctrough, ss (μg/mL)c 2.75 (101%) 1.70 (83.3%) Table 6: Mirikizumab-mrkz Estimated Systemic Exposure at Steady State in Subjects with Crohn's Disease a OMVOH 900 mg as an intravenous infusion over at least 90 minutes at Weeks 0, 4, and 8.
b OMVOH 300 mg as 2 subcutaneous injections (100 mg/mL and 200 mg/2 mL) at Week 12 and every 4 weeks thereafter up to Week 52.
c AUCtau, ss = area under the concentration-versus-time curve over one dosing interval at steady state; Cmax, ss = maximum concentration at steady state; Ctrough, ss = concentration at the end of the dosing interval at steady state; CV = geometric coefficient of variation.
OMVOH
900 mg Intravenous Infusiona
Geometric mean (CV%)OMVOH
300 mg Subcutaneous Injectionb
Geometric mean (CV%)Cmax, ss (μg/mL)c 332 (21%) 13.6 (48%) AUCtau, ss (μg*day/mL)c 1820 (38%) 220 (56%) Ctrough, ss (μg/mL)c 10.4 (108%) 2.52 (88%) Absorption
Following subcutaneous dosing of OMVOH for ulcerative colitis median (range) Tmax was 5 (3.08 to 6.75) days post dose and geometric mean (CV%) absolute bioavailability was 44% (34%).
Following subcutaneous dosing of OMVOH for Crohn's disease, median (range) Tmax was 5 (3 to 6.83) days post dose and geometric mean (CV%) absolute bioavailability was 36.3% (31%).
Injection site location (abdomen, upper arm, or thigh) did not significantly influence bioavailability of mirikizumab-mrkz following subcutaneous injection.
Distribution
In subjects with ulcerative colitis, the geometric mean (CV%) total volume of distribution was 4.83 L (21%).
In subjects with Crohn's disease, the geometric mean (CV%) total volume of distribution was 4.4 L (14%).
Metabolism/Elimination
Mirikizumab-mrkz is a humanized IgG4 monoclonal antibody and is expected to be degraded into small peptides and amino acids via catabolic pathways in the same manner as endogenous IgG.
In subjects with ulcerative colitis, the geometric mean (CV%) clearance was 0.0229 L/hours (34%) and the geometric mean (CV%) elimination half-life was 9.3 days (40%). Clearance is independent of dose.
In subjects with Crohn's disease, the geometric mean (CV%) clearance was 0.0202 L/hours (38%) and the geometric mean (CV%) elimination half-life was 9.3 days (26%). Clearance is independent of dose.
Specific Populations
There were no clinically significant differences in the pharmacokinetics of mirikizumab-mrkz based on age (18 to 79 years), sex, race (White or Asian), or mild and moderate renal impairment (i.e., estimated creatinine clearance by Cockcroft-Gault equation: 30 to 89 mL/min).
Body Weight
Following intravenous administration of 300 mg, the recommended induction dose, in subjects with ulcerative colitis weighing 90 kg or greater, the estimated geometric mean mirikizumab-mrkz average concentration (Cavg) was 20% lower compared with subjects weighing less than 90 kg. Following subcutaneous administration of 200 mg, the recommended maintenance dose, in subjects with ulcerative colitis weighing 90 kg or greater, the estimated geometric mean Cavg was 38% lower compared with subjects weighing less than 90 kg. In Study UC-2 (maintenance), the rate of clinical remission and clinical response did not differ significantly between subjects weighing 90 kg or greater and subjects weighing less than 90 kg.
Following intravenous administration of 900 mg, the recommended induction dose, in subjects with Crohn's disease weighing 90 kg or greater, the estimated geometric mean mirikizumab-mrkz Cavg was 13% lower compared with subjects weighing less than 90 kg. Following subcutaneous administration of 300 mg, the recommended maintenance dose, in subjects with Crohn's disease weighing 90 kg or greater, the estimated geometric mean Cavg was 34% lower compared with subjects weighing less than 90 kg. The rate of clinical remission and clinical response in Crohn's disease did not differ significantly between subjects weighing 90 kg or greater and subjects weighing less than 90 kg.
Drug Interaction Studies
Population pharmacokinetic analyses indicated that the clearance of OMVOH was not impacted by concomitant administration of aminosalicylates, corticosteroids, or oral immunomodulators (6-MP, AZA, MTX, tioguanine) in subjects with ulcerative colitis or Crohn's disease.
No drug-drug interaction studies were conducted in subjects with ulcerative colitis or Crohn's disease at the recommended dosage. Based on a clinical drug-drug interaction study conducted in subjects with another condition, multiple subcutaneous doses of 250 mg every 4 weeks of mirikizumab-mrkz did not result in changes in the exposure of midazolam (CYP3A substrate), warfarin (CYP2C9 substrate), dextromethorphan (CYP2D6 substrate), omeprazole (CYP2C19 substrate), or caffeine (CYP1A2 substrate).
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of mirikizumab-mrkz or of other mirikizumab products.
During the 52-week treatment period in studies UC-1 and UC-2, 23% (88/378) of OMVOH-treated subjects at the recommended dosage and evaluable for assessment, developed anti-mirikizumab-mrkz antibodies (referred to as anti-drug antibodies (ADA)). Of those who developed ADA, 33/88 (38%) developed titers ≥1:160. Of these 33 OMVOH-treated subjects, 10 had reduced serum trough concentrations of mirikizumab-mrkz compared to subjects who did not develop anti-mirikizumab-mrkz antibodies, and 5 of these 10 subjects did not achieve clinical response at Week 52. There is insufficient data to assess whether the observed ADA-associated pharmacokinetic changes reduced effectiveness. There is no identified clinically significant effect of ADA on the safety of OMVOH over the treatment duration of 52-weeks.
During the 52-week treatment period in study CD-1, 13% (79/622) of OMVOH-treated subjects at the recommended dosage and evaluable for assessment developed ADA. There is no identified clinically significant effect of ADA on the pharmacokinetics, effectiveness, or safety of OMVOH over the treatment duration of 52-weeks.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal studies have not been conducted to evaluate the carcinogenic or mutagenic potential of mirikizumab-mrkz.
No organ weight or histopathology effects were observed in the male or female reproductive tract in sexually mature cynomolgus monkeys that received subcutaneous mirikizumab-mrkz once weekly for 26 weeks, at a dose of 100 mg/kg (at least 2 times the MRHD of mirikizumab-mrkz, based on exposure comparisons).
-
14 CLINICAL STUDIES
14.1 Ulcerative Colitis
The safety and efficacy of OMVOH was evaluated in two randomized, double-blind, placebo-controlled clinical studies, one induction study [UC-1 (NCT03518086)] and one maintenance study [UC-2 (NCT03524092)], in adult subjects with moderately to severely active ulcerative colitis who had inadequate response, loss of response, or failed to tolerate any of the following: corticosteroids, 6-mercaptopurine, azathioprine, biologic therapy (TNF blocker, vedolizumab), or tofacitinib. The 12-week intravenous induction study (UC-1) was followed by the 40-week subcutaneous randomized withdrawal maintenance study (UC-2).
Study UC-1
In UC-1, efficacy was evaluated in 1062 subjects who were randomized 3:1 at Week 0 to receive 300 mg OMVOH or placebo by intravenous infusion at Week 0, Week 4, and Week 8. Subjects had a mean age of 43 years (range 18 to 79 years); 40% were female; and 71% identified as White, 25% as Asian, 1% as American Indian or Alaska Native, 1% as Black or African American, and <2% as another racial group or did not report their racial group. Subjects were permitted to use stable doses of aminosalicylates, immunomodulators (6-mercaptopurine, azathioprine, methotrexate), and oral corticosteroids (prednisone ≤20 mg/day or equivalent, extended-release budesonide 9 mg/day, beclomethasone dipropionate 5 mg/day). At baseline, 41% of subjects were receiving oral corticosteroids, 24% were receiving immunomodulators, and 75% were receiving aminosalicylates.
At baseline, 57% were biologic and Janus Kinase inhibitor (JAKi) naive, 41% had failed at least one biologic, 3% had failed a JAKi, and 2% had previously received but had not failed a biologic or JAKi.
Disease activity was assessed based on the modified Mayo score (mMS), which ranges from 0 to 9 and has three subscores that are each scored from 0 (normal) to 3 (most severe): stool frequency, rectal bleeding, and findings on centrally read endoscopy subscore. At baseline, subjects had a mMS of 5 to 9, including a centrally read endoscopy subscore of 2 or 3. An endoscopy subscore of 2 was defined by marked erythema, absent vascular pattern, friability, and erosions; and a subscore of 3 was defined by spontaneous bleeding and ulceration. Subjects had a median mMS of 7, and 58% had severely active disease (mMS of 7 to 9).
The primary endpoint was clinical remission at Week 12. The secondary endpoints were clinical response, endoscopic improvement, and histologic-endoscopic mucosal improvement (see Table 7).
Table 7: Proportion of Subjects with Ulcerative Colitis Meeting Efficacy Endpoints in UC-1 at Week 12 JAKi = Janus Kinase inhibitor
a OMVOH 300 mg as an intravenous infusion at Week 0, Week 4, and Week 8.
b Adjusted treatment difference based on Cochran-Mantel-Haenszel method adjusted for randomization stratification factors.
c Clinical remission based on mMS is defined as: stool frequency subscore = 0 or 1, rectal bleeding subscore = 0, and centrally read endoscopy subscore = 0 or 1 (excluding friability).
d Tested at an alpha level of 0.00125, with a p-value <0.001.
e Prior biologic or JAKi failure includes loss of response, inadequate response, or intolerance to one or more biologic therapy (TNF blocker or vedolizumab), or tofacitinib.
f Clinical response is defined as a decrease in the mMS of ≥2 points with ≥30% decrease from baseline, and either a decrease of ≥1 point in the rectal bleeding subscore from baseline or a rectal bleeding subscore of 0 or 1.
g Endoscopic improvement is defined as a centrally read endoscopy subscore of 0 or 1 (excluding friability).
h Histologic-endoscopic mucosal improvement is defined as achieving both endoscopic improvement (centrally read endoscopy subscore of 0 or 1, excluding friability) and histologic improvement (neutrophil infiltration in <5% of crypts, no crypt destruction, and no erosions, ulcerations, or granulation tissue based on the Geboes scoring system).
Endpoint Placebo OMVOH 300 mg Intravenous Infusiona Treatment Differenceb
(95% CI)Clinical remissionc Total Population N = 267
15%N = 795
24%10%d
(5, 15)Biologic and JAKi naive N = 155
18%N = 450
31%Prior biologic or JAKi failuree N = 107
8%N = 331
15%Clinical responsef Total Population N = 267
43%N = 795
65%22%d
(15, 28)Biologic and JAKi naive N = 155
52%N = 450
71%Prior biologic or JAKi failured, e N = 107
31%N = 331
56%Endoscopic improvementg Total Population N = 267
21%N = 795
34%14%d
(8, 20)Biologic and JAKi naive N = 155
28%N = 450
44%Prior biologic or JAKi failuree N = 107
10%N = 331
22%Histologic-endoscopic mucosal improvementh Total Population N = 267
14%N = 795
25%11%d
(6, 16)Biologic and JAKi naive N = 155
19%N = 450
34%Prior biologic or JAKi failuree N = 107
7%N = 331
13%Study UC-1 was not designed to evaluate the relationship of histologic-endoscopic mucosal improvement at Week 12 to disease progression and long-term outcomes.
Study UC-2
The maintenance study (UC-2) evaluated 506 subjects who achieved clinical response at Week 12 in Study UC-1. These subjects were randomized 2:1 to receive 200 mg OMVOH or placebo subcutaneously every 4 weeks for 40 weeks in UC-2, for a total of 52 weeks of treatment. Subjects who were on concomitant ulcerative colitis therapies during UC-1 were required to continue on stable doses of oral aminosalicylates and immunomodulators (6-mercaptopurine, azathioprine, methotrexate). Corticosteroid tapering was required for subjects who were receiving corticosteroids at baseline and achieved clinical response in UC-1.
The primary endpoint was clinical remission at Week 40. The secondary endpoints were endoscopic improvement, maintenance of clinical remission in subjects who achieved clinical remission at Week 12, corticosteroid-free clinical remission, and histologic-endoscopic mucosal improvement (see Table 8).
Table 8: Proportion of Subjects with Ulcerative Colitis Meeting Efficacy Endpoints in UC-2 at Week 40 (a total of 52 weeks of treatment) Endpoint Placeboa OMVOH
200 mg
Subcutaneous InjectionbTreatment Differencec
(95% CI)JAKi = Janus Kinase inhibitor
a The placebo arm includes subjects treated with OMVOH during the induction study (UC-1) and were randomized to receive placebo through Week 40.
b OMVOH 200 mg as a subcutaneous injection at Week 12 and every 4 weeks thereafter for up to an additional 40 weeks.
c Adjusted treatment difference (95% CI) based on Cochran-Mantel-Haenszel method adjusted for randomization stratification factors.
d Among subjects who achieved clinical response at Week 12 in UC-1 with OMVOH induction treatment.
e Clinical remission based on mMS is defined as: stool frequency subscore = 0 or 1, rectal bleeding subscore = 0, and centrally read endoscopy subscore = 0 or 1 (excluding friability).
f p<0.001.
g Prior biologic or JAKi failure includes loss of response, inadequate response, or intolerance to one or more biologic therapy (TNF blocker or vedolizumab), or tofacitinib.
h Endoscopic improvement is defined as a centrally read endoscopy subscore of 0 or 1 (excluding friability).
i Among subjects who achieved clinical remission at Week 12 in UC-1 with OMVOH induction treatment.
j p<0.01.
k Corticosteroid-free clinical remission is defined as clinical remission at Week 40 and no corticosteroid use for ≥12 weeks prior to Week 40 assessment.
l Histologic-endoscopic mucosal improvement is defined as achieving both endoscopic improvement (centrally read endoscopy subscore of 0 or 1, excluding friability) and histologic improvement (no neutrophils in crypts or lamina propria, no crypt destruction, and no erosions, ulcerations, or granulation tissue based on the Geboes scoring system).
Clinical remissiond, e Total Population N = 169
27%N = 337
51%22%f
(14, 31)Biologic and JAKi naive N = 109
33%N = 208
53%Prior biologic or JAKi failureg N = 59
15%N = 121
45%Endoscopic improvementd, h Total Population N = 169
30%N = 337
58%27%f
(19, 36)Biologic and JAKi naive N = 109
35%N = 208
62%Prior biologic or JAKi failureg N = 59
20%N = 121
50%Maintenance of clinical remission in patients who achieved clinical remission at Week 12i Total Population N = 62
40%N = 128
66%23%j
(8, 38)Biologic and JAKi naive N = 48
48%N = 91
66%Prior biologic or JAKi failureg N = 14
14%N = 34
65%Corticosteroid-free clinical remissiond, k Total Population N = 169
27%N = 337
50%22%f
(13, 30)Biologic and JAKi naive N = 109
33%N = 208
52%Prior biologic or JAKi failureg N = 59
15%N = 121
45%Histologic-endoscopic mucosal improvementd, l Total Population N = 169
22%N = 337
43%19%f
(11, 27)Biologic and JAKi naive N = 109
27%N = 208
47%Prior biologic or JAKi failureg N = 59
14%N = 121
36%Study UC-2 was not designed to evaluate the relationship of histologic-endoscopic mucosal improvement at Week 40 to disease progression and long-term outcomes.
Bowel Urgency
Bowel urgency was assessed during UC-1 and UC-2 with an Urgency Numeric Rating Scale (NRS) of 0 to 10. A greater proportion of subjects with a baseline Urgency NRS weekly average score ≥3 treated with OMVOH compared to placebo reported an Urgency NRS weekly average score of 0 or 1 (39% versus 23%) at Week 40. Urgency NRS weekly average scores of 0 to 1 were also observed in a greater proportion of subjects treated with OMVOH compared to placebo at Week 12.
14.2 Crohn's Disease
The safety and efficacy of OMVOH was evaluated in a randomized, double-blind, placebo-controlled study [CD-1 (NCT03926130)] in adult subjects with moderately to severely active Crohn's disease who had an inadequate response, loss of response, or intolerance to corticosteroids, immunomodulators (azathioprine, 6-mercaptopurine, and methotrexate), and/or biologics (TNF blockers, integrin receptor antagonists).
In CD-1, the efficacy population consisted of 679 subjects who were randomized 3:1 at Week 0 to receive OMVOH 900 mg by intravenous infusion at Week 0, Week 4, and Week 8 followed by a dosage of 300 mg by subcutaneous injection at Week 12 and then every 4 weeks for 40 weeks, or placebo. Subjects had a mean age of 36 years (range 18 to 74 years); 42% were female; and 71% identified as White, 25% as Asian, <1% as American Indian or Alaska Native, 1% as Black or African American, and 2% as another racial group or did not report their racial group. Subjects were permitted to use stable doses of oral corticosteroids (prednisone ≤30 mg/day or equivalent, extended-release budesonide 9 mg/day), immunomodulators (6-mercatopurine, azathioprine, or methotrexate) and/or aminosalicylates. At baseline, 31% of subjects were receiving oral corticosteroids, 26% were receiving immunomodulators, and 44% were receiving aminosalicylates.
At baseline, 47% had a loss of response, inadequate response, or intolerance to one or more biologic therapy.
Disease activity at baseline was assessed by the Crohn's Disease Activity Index (CDAI) and the Simple Endoscopic Score for Crohn's disease (SES-CD). Moderately to severely active CD was defined by a CDAI of ≥220 and an SES-CD ≥7 (centrally read) for subjects with ileal-colonic disease or ≥4 for subjects with isolated ileal disease. At baseline, subjects had a median CDAI of 329 and SES-CD of 12.
The coprimary endpoints of clinical remission by CDAI and endoscopic response by SES-CD were assessed at Week 52. Secondary efficacy endpoints included endoscopic response at Week 12 and endoscopic remission and corticosteroid-free clinical remission at Week 52 (see Table 9).
Table 9: Proportion of Subjects with Crohn's Disease Meeting Efficacy Endpoints in CD-1 CI = confidence interval
a The placebo group includes all 168 subjects randomized to placebo at baseline. Of those, 67 (40%) subjects who did not achieve clinical response by patient-reported outcome at Week 12 were switched to treatment with OMVOH and their efficacy data are included here with the remaining subjects randomized to placebo who did not receive OMVOH.
b Following OMVOH 900 mg as an intravenous infusion at Week 0, Week 4, and Week 8, subjects received OMVOH 300 mg as a subcutaneous injection at Week 12 and every 4 weeks thereafter for up to an additional 40 weeks.
c Adjusted treatment difference was based on Cochran-Mantel-Haenszel method adjusted for randomization stratification factors.
d Clinical remission is defined as CDAI <150.
e p-value <0.001.
f Prior biologic failure includes loss of response, inadequate response, or intolerance to one or more biologic therapy (TNF blockers, and integrin receptor antagonists).
g Endoscopic response is defined as >50% reduction from baseline in SES-CD total score, based on central reading.
h Endoscopic remission is defined as SES-CD total score ≤4 and at least a 2-point reduction from baseline, with no segment subscore >1, based on central reading.
i Corticosteroid-free clinical remission is defined as subjects who were corticosteroid-free from Week 40 to Week 52, and had a CDAI <150 at Week 52.
Placeboa OMVOHb Treatment Differencec
(95% CI)Coprimary Endpoints Clinical remissiond at Week 52 Total population N = 168
36%N = 511
53%17%e
(9%, 25%)Without prior biologic failure N = 89
45%N = 268
56%Prior biologic failuref N = 79
25%N = 243
49%Endoscopic responseg at Week 52 Total population N = 168
23%N = 511
46%23%e
(15%, 30%)Without prior biologic failure N = 89
27%N = 268
49%Prior biologic failuref N = 79
18%N = 243
43%Additional Endpoints Endoscopic responseg at Week 12 Total population N = 168
11%N = 511
32%22%e
(16%, 28%)Without prior biologic failure N = 89
12%N = 268
37%Prior biologic failuref N = 79
9%N = 243
28%Corticosteroid-free clinical remissioni at Week 52 Total population N = 168
35%N = 511
50%16% e
(7%, 24%)Without prior biologic failure N = 89
43%N = 268
54%Prior biologic failuref N = 79
25%N = 243
46%Endoscopic remissionh at Week 52 Total population N = 168
8%N = 511
19%11% e
(6%, 16%)Without prior biologic failure N = 89
10%N = 268
22%Prior biologic failuref N = 79
5%N = 243
16%Stool Frequency and Abdominal Pain
In CD-1, reductions in abdominal pain were observed as early as Week 6 and in stool frequency as early as Week 12 in subjects treated with OMVOH compared to placebo.
Fatigue
In CD-1, subjects treated with OMVOH experienced a clinically meaningful improvement in fatigue, assessed by the change from baseline in the Functional Assessment of Chronic Illness Therapy Fatigue Scale (FACIT-Fatigue), at Week 12, compared to placebo-treated subjects. The effect of OMVOH to improve fatigue after 12 weeks has not been established.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
OMVOH (mirikizumab-mrkz) injection is a sterile, preservative-free, clear to opalescent, colorless to slightly yellow to slightly brown solution for intravenous infusion or subcutaneous injection.
OMVOH is supplied in indication specific packaging as:
Table 10: Packaging Information for OMVOH Presentation Indication Package Size NDC Code Single-dose Vial 300 mg/15 mL (20 mg/mL) Ulcerative colitis and Crohn's disease
Carton of 1 0002-7575-01 Single-dose Prefilled Pen 100 mg/mL + 100 mg/mL Ulcerative colitis Carton of 2 0002-8011-27 200 mg/2 mL Ulcerative colitis Carton of 1 0002-3116-11 200 mg/2 mL + 100 mg/mL Crohn's disease
Carton of 2
(1 of each)0002-7717-11 Single-dose Prefilled Syringe 100 mg/mL + 100 mg/mL Ulcerative colitis Carton of 2 0002-8870-27 200 mg/2 mL (100 mg/mL) Ulcerative colitis Carton of 1 0002-1442-11 200 mg/2 mL (100 mg/mL) + 100 mg/mL Crohn's disease Carton of 2
(1 of each)0002-7722-11 Note to Pharmacist: The entire carton of 2 prefilled pen or 2 prefilled syringes are to be dispensed as a unit.
Each 100 mg/mL single-dose prefilled pen or prefilled syringe consists of a 1 mL glass syringe with a fixed 27-gauge ½ inch needle.
Each 200 mg/2 mL single-dose prefilled pen or 200 mg/2 mL (100 mg/mL) prefilled syringe consists of a 2 mL glass syringe with a fixed 27-gauge 8 mm needle.
Storage and Handling
- Store refrigerated at 2°C to 8°C (36°F to 46°F).
- Do not freeze. Do not use OMVOH if it has been frozen.
- Do not shake.
- Keep OMVOH in the original carton to protect from light until the time of use.
- OMVOH is sterile and preservative-free. Discard any unused portion.
- If needed, the prefilled pen or prefilled syringe may be stored at room temperature up to 30°C (86°F) for up to 2 weeks in the original carton to protect from light. Once OMVOH has been stored at room temperature, do not return to the refrigerator. If these conditions are exceeded, OMVOH must be discarded.
- The vial, prefilled pen, and prefilled syringe are not made with dry natural rubber latex.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient and/or caregiver to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Hypersensitivity Reactions
Advise patients to discontinue OMVOH and seek immediate medical attention if they experience any symptoms of serious hypersensitivity reactions [see Warnings and Precautions (5.1)].
Infections
Advise patients that OMVOH may lower the ability of their immune system to fight infections and to contact their healthcare provider immediately if they develop any symptoms of infection [see Warnings and Precautions (5.2)].
Tuberculosis
Advise patients to contact their healthcare provider if they experience symptoms suggestive of TB (e.g., unexplained fever, cough, or difficulty breathing) [see Warnings and Precautions (5.3)].
Hepatotoxicity
Inform patients that OMVOH may cause liver injury. Advise patients to seek immediate medical attention if they experience symptoms suggestive of liver dysfunction (e.g., unexplained rash, nausea, vomiting, abdominal pain, fatigue, anorexia, or jaundice and/or dark urine) [see Warnings and Precautions (5.4)].
Immunizations
Advise patients that vaccination with live vaccines is not recommended during OMVOH treatment and immediately prior to or after OMVOH treatment. Medications that interact with the immune system may increase the risk of infection following administration of live vaccines. Instruct patients to inform their healthcare provider that they are taking OMVOH prior to receiving a vaccination [see Warnings and Precautions (5.5)].
Pregnancy
Advise patients who are exposed to OMVOH during pregnancy to contact Eli Lilly and Company [see Use in Specific Populations (8.1)].
Administration
Instruct patients in preparation and administration of OMVOH, including choosing anatomical sites for subcutaneous administration, and proper subcutaneous injection technique. Instruct patients in the technique of prefilled pen or prefilled syringe disposal [see Instructions for Use].
Instruct ulcerative colitis patients or caregivers to administer either:
- Two 100 mg prefilled pens or two 100 mg prefilled syringes to achieve the full 200 mg dose of OMVOH.
- One 200 mg prefilled pen or one 200 mg prefilled syringe to achieve the full 200 mg dose of OMVOH.
Instruct Crohn's disease patients or caregivers to administer a 100 mg prefilled pen or prefilled syringe and a 200 mg prefilled pen or prefilled syringe in any order to achieve the full 300 mg dose of OMVOH.
Eli Lilly and Company, Indianapolis, IN 46285, USA
US License No. 1891Copyright © 2023, 2025, Eli Lilly and Company. All rights reserved.
Pat.: www.lilly.com/patents
OMV-0009-USPI-20251104
-
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised: 10/2025
Medication Guide
OMVOHTM (ahm-VOH)
(mirikizumab-mrkz)
injection, for intravenous or subcutaneous useWhat is the most important information I should know about OMVOH?
OMVOH can cause serious side effects, including:
-
Serious allergic reactions. OMVOH may cause serious allergic reactions that may need to be treated in a hospital and may be life-threatening. Stop using OMVOH and get emergency medical help right away if you develop any of the following symptoms of a serious allergic reaction:
- fainting, dizziness, feeling lightheaded (low blood pressure)
- swelling of your face, eyelids, lips, mouth, tongue, throat, or trouble swallowing
- trouble breathing, throat tightening or wheezing
- chest tightness
- fast heartbeat or pounding in your chest (tachycardia)
- severe itching, hives, or redness all over your body
- sweating
-
Infections. OMVOH may lower the ability of your immune system to fight infections and may increase your risk of infections. Your healthcare provider should not start treatment with OMVOH until your infection is gone.
- Before starting your treatment with OMVOH, your healthcare provider should test you for tuberculosis (TB).
- If your healthcare provider feels that you are at risk for TB, you may be treated with medicine for TB before you begin treatment with OMVOH.
- Your healthcare provider should watch you closely for signs and symptoms of TB while you are being treated with OMVOH and after treatment.
- Before starting OMVOH, tell your healthcare provider if you think you have an infection or have symptoms of an infection such as:
- fever, sweating, or chills
- muscle aches and pain
- cough or shortness of breath
- blood in your mucus (phlegm)
- flu-like symptoms
- headache
- warm, red, or painful skin or sores on your body
- diarrhea or stomach pain
- weight loss
- nausea or vomiting
- pain during urination
After starting OMVOH, tell your healthcare provider right away if you have any symptoms of an infection.
-
Liver problems. OMVOH may cause liver problems. Your healthcare provider will do blood tests to check your liver enzyme and bilirubin levels before treatment, for at least 24 weeks during treatment, and possibly after treatment with OMVOH. Your healthcare provider may hold or stop your treatment if needed. Tell your healthcare provider right away if you develop any signs and symptoms of liver problems, including:
- unexplained rash
- nausea
- vomiting
- stomach-area (abdominal) pain
- feeling tired
- loss of appetite
- yellowing of the skin or the whites of your eyes
- dark urine
What is OMVOH?
OMVOH is a prescription medicine used to treat:
- adults with moderately to severely active ulcerative colitis
- adults with moderately to severely active Crohn's disease
It is not known if OMVOH is safe and effective in children. Do not use OMVOH if you:
- are allergic to mirikizumab-mrkz or any of the ingredients in OMVOH. See the end of this Medication Guide for a complete list of ingredients in OMVOH.
Before you use OMVOH, tell your healthcare provider about all your medical conditions, including if you:
- have any of the conditions or symptoms listed in the section “What is the most important information I should know about OMVOH?”
- have recently received or are scheduled to receive any vaccinations. Medicines that affect your immune system may increase your risk of getting an infection after receiving live vaccines.
- You should be brought up to date with all age required vaccines before starting treatment with OMVOH.
- You should avoid receiving ‘live’ vaccines right before, during, or right after treatment with OMVOH. Tell your healthcare provider that you are taking OMVOH before receiving a vaccine.
- are pregnant, or plan to become pregnant. It is not known if OMVOH will harm your unborn baby. There will be a pregnancy registry to collect information about women who are exposed to OMVOH during pregnancy. If you become pregnant while taking OMVOH, you are encouraged to report your pregnancy to Eli Lilly and Company at 1-800-Lilly-Rx (1-800-545-5979).
- are breastfeeding or plan to breastfeed. It is not known if OMVOH passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby while using OMVOH.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. How should I use OMVOH?
- Use OMVOH exactly as your healthcare provider tells you to.
- You will receive your first 3 doses of OMVOH through a vein in your arm (intravenous infusion) in a healthcare facility by a healthcare provider every 4 weeks.
- For ulcerative colitis, each infusion will last about 30 minutes.
- For Crohn's disease, each infusion will last about 90 minutes.
- After your intravenous infusions, you will continue to receive OMVOH as an injection under the skin (subcutaneous injection) every 4 weeks as described below.
- See the detailed Instructions for Use that comes with OMVOH for information on how to prepare and inject a dose of OMVOH, and how to properly throw away (dispose of) used OMVOH prefilled pens or prefilled syringes.
- OMVOH comes as 2 different types of 1-time use devices:
- a prefilled pen,
- a prefilled syringe.
Your healthcare provider will decide which type of device is best for you.
- For ulcerative colitis, you will need to inject one 200 mg/2 mL prefilled pen or 200 mg/2mL (100 mg/mL) prefilled syringe, or two 100 mg/mL prefilled pens or prefilled syringes for your full dose.
- If 2 injections are required for your full dose, inject 1 OMVOH prefilled pen or prefilled syringe followed right away by the other OMVOH prefilled pen or prefilled syringe.
- For Crohn's disease, you will need 2 injections either with 2 prefilled pens or 2 prefilled syringes for your full dose.
- You will need to inject one OMVOH 100 mg/mL prefilled pen or prefilled syringe and one 200 mg/2 mL prefilled pen or 200 mg/2 mL (100 mg/mL) prefilled syringe in any order.
-
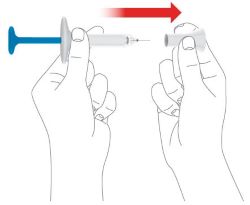
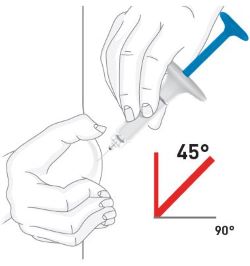
Injecting OMVOH under your skin:
- OMVOH is intended for use under the guidance and supervision of your healthcare provider. If your healthcare provider decides that you or a caregiver may give your injections of OMVOH at home, you should receive training on the correct way to prepare and inject OMVOH. Do not try to inject OMVOH yourself until you or your caregiver have been shown how to inject OMVOH.
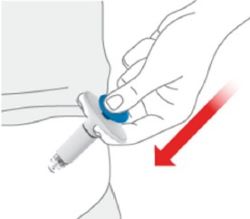
- Inject OMVOH under the skin in your stomach area (abdomen), upper legs (thighs), or back of the upper arms.
- Do not give an injection in an area that is tender, bruised, red, or hard.
- Use a different injection site each time you use OMVOH.
- If you miss a dose of OMVOH, inject the missed dose as soon as possible. Then take your next dose in 4 weeks. If you have questions about how often you should use OMVOH, talk to your healthcare provider.
What are the possible side effects of OMVOH?
OMVOH can cause serious side effects, including:
The most common side effects of OMVOH in people treated for ulcerative colitis include: - upper respiratory infections
- injection site reactions
- joint pain
- headache
- rash
- herpes viral infections
The most common side effects of OMVOH in people treated for Crohn's disease include:
- upper respiratory infections
- injection site reactions
- elevated liver blood tests
- headache
- joint pain
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of OMVOH. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store OMVOH?
- In the original carton, store OMVOH prefilled pens and prefilled syringes in a refrigerator between 36°F to 46°F (2°C to 8°C).
- Do not freeze. Do not use OMVOH if it has been frozen.
- Do not shake.
- Keep OMVOH in the original carton to protect from light until the time of use.
- If needed, your prefilled pens or prefilled syringes may be stored at room temperature for up to 2 weeks in the original carton. Do not store above 86°F (30°C).
- When OMVOH has been stored at room temperature, do not return it to the refrigerator.
-
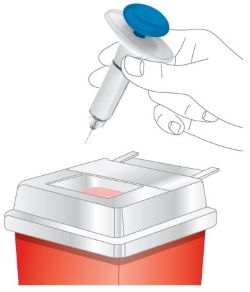
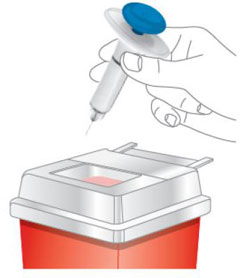
Do not use and throw away (dispose of) your prefilled pens and prefilled syringes if they:
- have been frozen.
- have been shaken.
- have not been protected from light in the original carton.
- have been stored at room temperature more than 2 weeks.
Keep OMVOH and all medicines out of the reach of children. General information about the safe and effective use of OMVOH.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use OMVOH for a condition for which it was not prescribed. Do not give OMVOH to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about OMVOH that is written for health professionals.What are the ingredients in OMVOH?
Active ingredient: mirikizumab-mrkz.
Inactive ingredients:
- Intravenous infusion: anhydrous citric acid, polysorbate 80, sodium chloride, sodium citrate, and Water for Injection. Some formulations may also contain histidine, L-histidine hydrochloride monohydrate, and mannitol.
- Subcutaneous injection: histidine, L-histidine hydrochloride monohydrate, mannitol, polysorbate 80, sodium chloride, and Water for Injection.
OMVOH prefilled pens and prefilled syringes are not made with dry natural rubber latex.
OMVOHTM is a trademark of Eli Lilly and Company.
Eli Lilly and Company, Indianapolis, IN 46285, USA
US License No. 1891
Copyright © 2023, 2025, Eli Lilly and Company. All rights reserved.
For more information, go to www.OMVOH.com or call 1-800-545-5979.OMV-0005-MG-20251027
-
Serious allergic reactions. OMVOH may cause serious allergic reactions that may need to be treated in a hospital and may be life-threatening. Stop using OMVOH and get emergency medical help right away if you develop any of the following symptoms of a serious allergic reaction:
-
100 MG PREFILLED PEN INSTRUCTIONS FOR USE
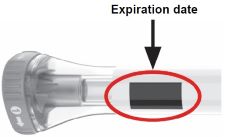
Storing OMVOH Prefilled Pens Refrigeration - In the original carton, store your prefilled Pens in the refrigerator between 36ºF to 46ºF (2ºC to 8ºC) until the expiration date.
- Do not freeze. Do not use OMVOH if it has been frozen.
Room temperature- If needed, your prefilled Pens may be stored at room temperature for up to 2 weeks in the original carton. Do not store above 86°F (30°C).
- When OMVOH has been stored at room temperature, do not return it to the refrigerator.
- Throw away (dispose of) OMVOH if not used within 2 weeks at room temperature.
- Protect your prefilled Pens from light until use.
Do not use the prefilled Pens and throw away (see Throwing away (disposing of) OMVOH Prefilled Pens) if they have been:- Frozen
- microwaved
- warmed with hot water
- left in direct sunlight
- shaken
- Keep OMVOH and all medicines out of the reach of children.
Read the Medication Guide for OMVOH inside this box to learn more about your medicine. This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Eli Lilly and Company
Indianapolis, IN 46285, USA
US License Number 1891
OMVOH™ is a trademark of Eli Lilly and Company.
Copyright © 2023, 2025, Eli Lilly and Company. All rights reserved.
Revised: October 2025
The OMVOH prefilled Pen meets the current dose accuracy and functional requirements of ISO 11608-1 and 11608-5.
OMV-0005-IFU-PEN-20251023
-
100 MG PREFILLED SYRINGE INSTRUCTIONS FOR USE
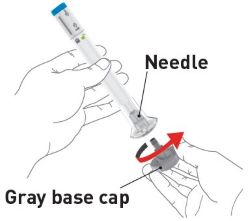
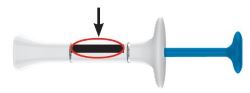
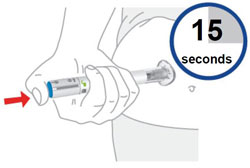
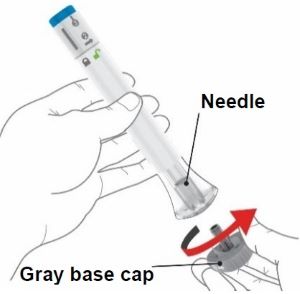
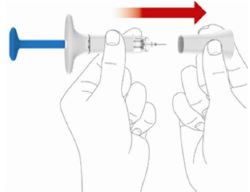
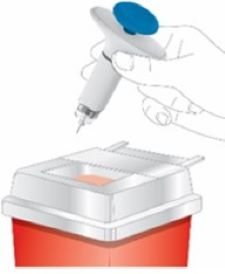
2 injections are required for a full dose. Inject one prefilled syringe immediately followed by the other prefilled syringe. 4 Inject the second prefilled syringe
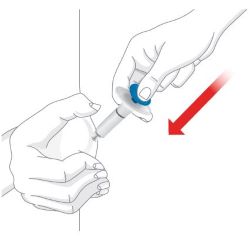
- Choose a new injection site at least 2 inches away and clean it.
- With your second prefilled syringe, repeat steps 1 to 3 right away after your first injection
- You must inject 2 prefilled syringes to complete your full 200 mg dose.
Storing OMVOH prefilled syringes - Store your prefilled syringes in the refrigerator between 36ºF to 46ºF (2ºC to 8ºC).
- If needed, your prefilled syringes may be stored at room temperature for up to 2 weeks. Do not store above 86°F (30°C). Throw away (dispose of) OMVOH if not used within 2 weeks at room temperature. When OMVOH has been stored at room temperature, do not return it to the refrigerator.
- Do not freeze your prefilled syringes.
- Store your prefilled syringes in the original carton to protect from light until use.
- Do not microwave your prefilled syringes, or run hot water over them, or leave them in direct sunlight.
- Do not shake your prefilled syringes.
- Throw away (dispose of) your prefilled syringes if any of the above conditions are not followed.
- Keep your prefilled syringes and all medicines out of the sight and reach of children.
Read the Medication Guide for OMVOH inside this box to learn more about your medicine. This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Eli Lilly and Company
Indianapolis, IN 46285, USA
US License Number 1891OMVOHTM is a trademark of Eli Lilly and Company.
Copyright © 2024, 2025, Eli Lilly and Company. All rights reserved.
Revised: October 2025
OMV-0003-IFU-PFS-20251023
-
100 MG + 200 MG PREFILLED PEN INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
OMVOHTM (ahm-voh)
(mirikizumab-mrkz)
injection, for subcutaneous use
Prefilled Pens
One 200 mg/2 mL Prefilled Pen and one 100 mg/mL Prefilled Pen for a full dose of 300 mg
This Instructions for Use contains information on how to inject OMVOH.
Before you use the OMVOH prefilled Pens, read and carefully follow all the step-by-step instructions.
Important information you need to know before injecting OMVOH
- 2 OMVOH injections, using the provided 100 mg/mL and 200 mg/2 mL prefilled Pens, are required for a full 300 mg dose.
- The prefilled Pens may be used in any order.
- Inject 1 OMVOH prefilled Pen followed right away by the other OMVOH prefilled Pen.
- Your healthcare provider should show you how to prepare and inject OMVOH using the prefilled Pens. Do not inject yourself or someone else until you have been shown how to inject OMVOH.
- Keep this Instructions for Use and read it as needed.
- Each OMVOH prefilled Pen is for one-time use only.
- The OMVOH prefilled Pen contains glass parts. Handle it carefully. If you drop it on a hard surface, do not use it. Use a new OMVOH prefilled Pen for your injection.
- Your healthcare provider can help you decide where on your body to inject your dose. Read the Choose and clean your injection sites section of these instructions to help you choose which area can work best for you.
- If you have vision or hearing problems, do not use OMVOH prefilled Pens without help from a caregiver.
- See Storing OMVOH Prefilled Pens for important storage information.
INSTRUCTIONS FOR USE
Before you use the OMVOH prefilled Pens, read and carefully follow all the step-by-step instructions.
2 Prefilled Pens = full 300 mg dose
After your first injection, choose a new injection site at least 2 inches (5 centimeters) away and clean it.
With your second prefilled Pen, repeat steps 1 to 3 right away after your first injection.
You must inject 2 prefilled Pens to complete your full 300 mg dose.
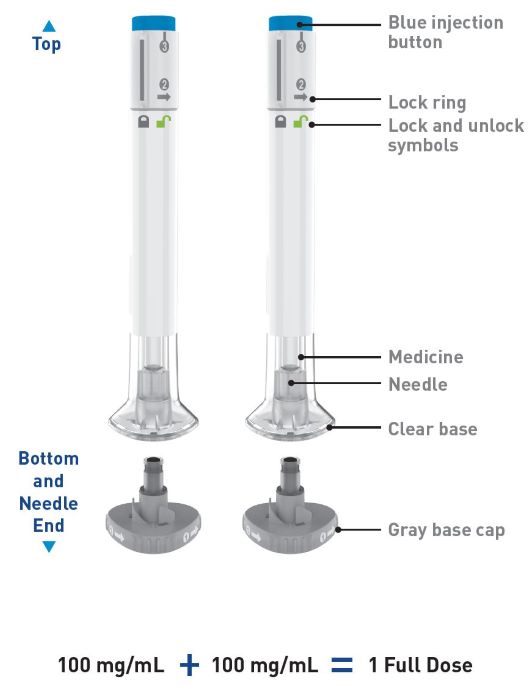
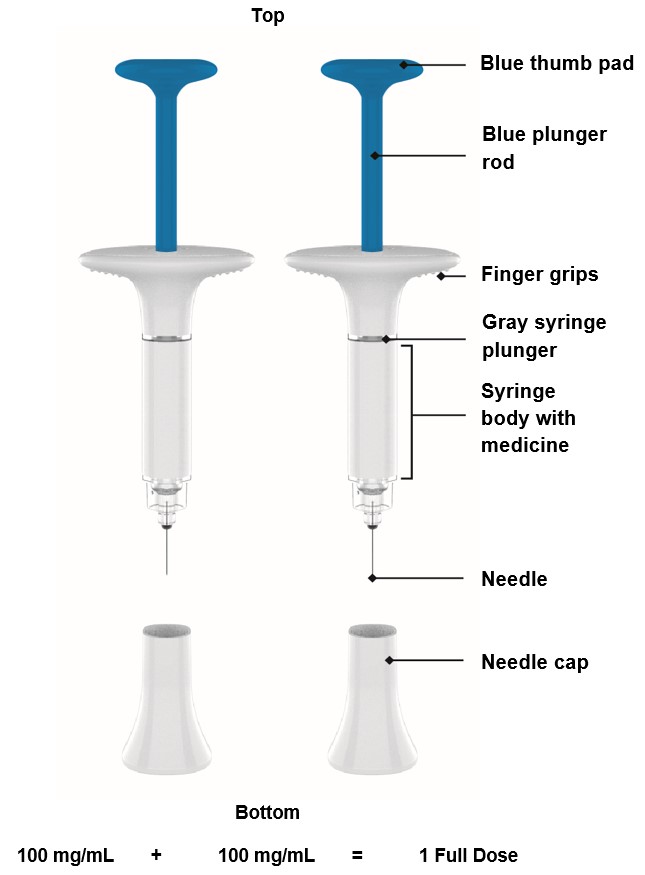
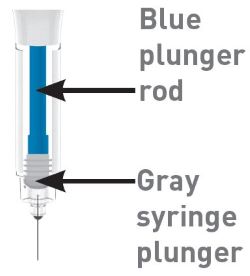
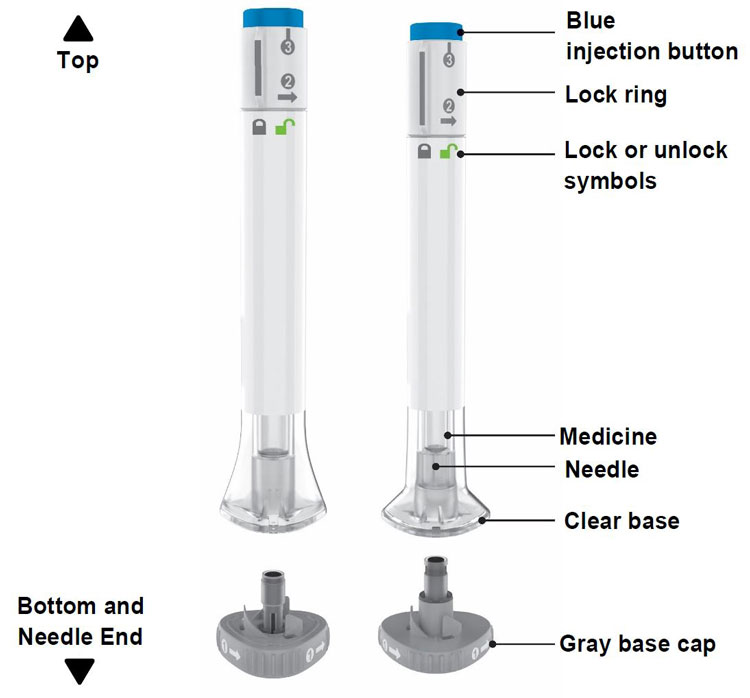
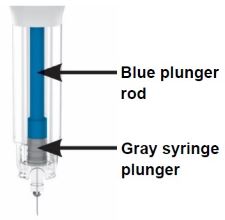
Parts of the OMVOH Prefilled Pens
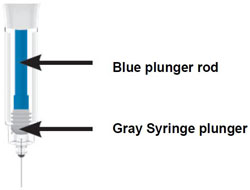
Inject both prefilled Pens in any order for a full 300 mg dose.
The 200 mg/2 mL prefilled Pen is larger than the 100 mg/mL prefilled Pen.
Preparing to inject OMVOH
Gather supplies
- a carton containing 2 OMVOH prefilled Pens from the refrigerator
- 2 alcohol wipes
- 2 cotton balls
- sharps disposal container
Inspect the prefilled Pens and the medicine
Remove both prefilled Pens from the carton.
Make sure you have the right medicine.
The medicine inside should be clear. It may be colorless to slightly yellow to slightly brown.
Wait 45 minutes
Do not warm up the prefilled Pens with a microwave, hot water, or direct sunlight.
With the gray base cap on, allow the prefilled Pens to warm up to room temperature for 45 minutes before injecting.
Choose and clean your injection sites
Your healthcare provider can help you choose the injection sites that are best for you. Clean the injection sites with an alcohol wipe and let them dry.
Injecting OMVOH
1. Uncap the first prefilled Pen (the prefilled Pens may be used in any order)
2. Place and unlock
Clear base
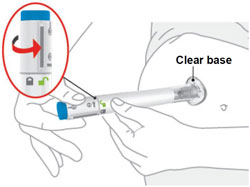

Place and hold the clear base flat against the skin.
Keep the clear base on the skin, then turn the lock ring to the unlock position.3. Press and hold for 15 seconds
4. Inject the second prefilled Pen
Choose a new injection site at least 2 inches away and clean it.
With your second prefilled Pen, repeat steps 1 to 3 right away after your first injection.
You must inject 2 prefilled Pens to complete your full 300 mg dose.
Throwing away (disposing of) OMVOH Prefilled Pens
Throw away both used prefilled Pens
Storing OMVOH Prefilled Pens
Refrigeration
- In the original carton, store your prefilled pens in the refrigerator between 36°F to 46°F (2°C to 8°C) until the expiration date.
- Do not freeze. Do not use OMVOH if it has been frozen.
Room Temperature
- If needed, your prefilled Pens may be stored at room temperature for up to 2 weeks in the original carton. Do not store above 86°F (30°C).
- When OMVOH has been stored at room temperature, do not return it to the refrigerator.
- Throw away (dispose of) OMVOH if not used within 2 weeks at room temperature.
- Protect your prefilled Pens from light until use.
Do not use the prefilled Pens and throw away (see Throwing away (disposing of) OMVOH Prefilled Pens) if they have been:
- frozen
- microwaved
- warmed with hot water
- left in direct sunlight
- shaken
Keep OMVOH and all medicines out of the reach of children.
Commonly asked questions
Q. The prefilled Pens are different sizes. Should I take one before the other? A. You can inject the prefilled Pens in any order. Inject both prefilled Pens for a full 300 mg dose. Q. What if I see air bubbles in the prefilled Pens? A. Air bubbles are normal. They will not harm you or affect your dose. Q. What if there is a drop of liquid on the tip of the needle when I remove the gray base cap? A. A drop of liquid on the tip of the needle is normal. This will not harm you or affect your dose. Do not touch the needle. Q. What if I unlock the prefilled Pen and press the blue injection button before twisting off the gray base cap? A. Do not remove the gray base cap. Throw away (dispose of) the prefilled Pen and get a new one. Q. Do I need to hold the blue injection button down until the injection is complete? A. You do not need to hold the blue injection button down, but it may help you keep the prefilled Pen steady against your skin. Q. What if the needle did not retract after my injection? A. Do not touch the needle or replace the gray base cap. Store the prefilled Pen in a safe place to avoid an accidental needlestick and contact 1-800-Lilly-Rx (1-800-545-5979) for instructions on how to return the prefilled Pen. Q. What if there is a drop of liquid or blood on my skin after my injection? A. This is normal. Press a cotton ball over the injection site. Do not rub the injection site. Q. How can I tell if my injection is complete? A. After you press the blue injection button, you will hear 2 loud clicks. The second loud click tells you that your injection is complete. You will also see the gray plunger at the top of the clear base. The injection may take up to 15 seconds. Q. What if I remove the prefilled Pen before the second loud click or before the gray plunger stops moving? A. You may not have received your full dose. Do not give another injection. Call your healthcare provider for help. Q. What if I heard more than 2 clicks during my injection – 2 loud clicks and 1 soft one. Did I get my complete injection? A. Some people may hear a soft click right before the second loud click. That is the normal operation of the prefilled Pen. Do not remove the prefilled Pen from your skin until you hear the second loud click. Additional Information:
If you have more questions about how to use the OMVOH prefilled Pens:
- Call your healthcare provider
- Call 1-800-Lilly-Rx (1-800-545-5979)
- Visit www.OMVOH.com

Scan this code to launch www.OMVOH.COM Used OMVOH Prefilled Pen disposal
Put the used OMVOH prefilled Pens in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) the OMVOH prefilled Pens in your household trash.
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container.
There may be state or local laws about how you should throw away needles and syringes.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
Do not recycle your used sharps disposal container.
Read the Medication Guide for OMVOH inside this box to learn more about your medicine.
Manufactured by:
Eli Lilly and Company
Indianapolis, IN 46285, USA
US License Number 1891OMVOHTM is a trademark of Eli Lilly and Company.
Copyright © 2025, Eli Lilly and Company. All rights reserved.
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Approved: January 2025
The OMVOH prefilled Pen meets the current dose accuracy and functional requirements of ISO 11608-1 and 11608-5.
OMV-0001-300MG-PEN-IFU-20250115
-
100 MG + 200 MG PREFILLED SYRINGE INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
OMVOHTM (ahm-voh)
(mirikizumab-mrkz)
injection, for subcutaneous use
Prefilled Syringes
One 200 mg/2 mL (100 mg/mL) Prefilled Syringe and One 100 mg/mL Prefilled Syringe for a Full Dose of 300 mg.
This Instructions for Use contains information on how to inject OMVOH.
Before you use the OMVOH prefilled Syringes, read and carefully follow all the step-by-step instructions.
Important information you need to know before injecting OMVOH
- 2 OMVOH injections, using the provided 100 mg/mL and 200 mg/2 mL (100 mg/mL) prefilled Syringes, are required for a full 300 mg dose.
- The prefilled Syringes may be used in any order.
- Inject 1 OMVOH prefilled Syringe followed right away by the other OMVOH prefilled Syringe.
- Your healthcare provider should show you how to prepare and inject OMVOH using the prefilled Syringes. Do not inject yourself or someone else until you have been shown how to inject OMVOH.
- Keep this Instructions for Use and read it as needed.
- Each OMVOH prefilled Syringe is for one-time use only.
- Your healthcare provider can help you decide where on your body to inject your dose. Read the Choose and clean your injection sites section of these instructions to help you choose which area can work best for you.
- If you have vision problems, do not use OMVOH prefilled Syringes without help from a caregiver.
- See Storing OMVOH prefilled Syringes for important storage information.
INSTRUCTIONS FOR USE
Before you use the OMVOH prefilled Syringes, read and carefully follow all the step-by-step instructions.
2 Prefilled Syringes = full 300 mg dose
After your first injection, choose a new injection site at least 2 inches away and clean it.
With your second prefilled Syringe, repeat steps 1 to 3 right away after your first injection.
You must inject 2 prefilled Syringes to complete your full 300 mg dose.
Parts of the OMVOH prefilled Syringes
Inject both prefilled Syringes in any order for a full 300 mg dose.
The 200 mg/2 mL (100 mg/mL) prefilled Syringe is larger than the 100 mg/mL prefilled Syringe.
Preparing to inject OMVOH
Gather supplies
- a carton containing 2 OMVOH prefilled Syringes from the refrigerator
- 2 alcohol wipes
- 2 cotton balls
- sharps disposal container
Inspect the prefilled Syringes and the medicine
Remove both prefilled Syringes from the carton.
Make sure you have the right medicine.
The medicine inside should be clear. It may be colorless to slightly yellow to slightly brown.
Wait 45 minutes
Do not warm up the prefilled Syringes with a microwave, hot water, or direct sunlight.
With the needle cap on, allow the prefilled Syringes to warm up to room temperature for 45 minutes before injecting.
Choose and clean your injection sites
Your healthcare provider can help you choose the injection sites that are best for you. Clean the injection sites with an alcohol wipe and let them dry.
Injecting OMVOH
1. Uncap the first prefilled Syringe (the prefilled Syringes may be used in any order)
2. Pinch the injection site and insert the needle
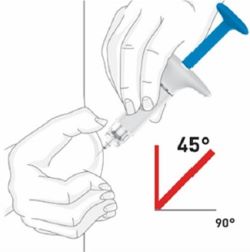
Gently pinch and hold a fold of skin where you will inject.
Insert the needle at a 45-degree angle.
Gently let go of the skin while keeping the needle in place.3. Inject the medicine
4. Inject the second prefilled Syringe
Choose a new injection site at least 2 inches away and clean it.
With your second prefilled Syringe, repeat steps 1 to 3 right away after your first injection.
You must inject 2 prefilled Syringes to complete your full 300 mg dose.
Throwing away (disposing of) OMVOH Prefilled Syringes
Throw away both used prefilled Syringes
Storing OMVOH Prefilled Syringes
Refrigeration
- In the original carton, store your prefilled Syringes in the refrigerator between 36°F to 46°F (2°C to 8°C) until the expiration date.
- Do not freeze. Do not use OMVOH if it has been frozen.
Room Temperature
- If needed, your prefilled Syringes may be stored at room temperature for up to 2 weeks in the original carton. Do not store above 86°F (30°C).
- When OMVOH has been stored at room temperature, do not return it to the refrigerator.
- Throw away (dispose of) OMVOH if not used within 2 weeks at room temperature.
- Protect your prefilled Syringes from light until use.
Do not use the prefilled Syringes and throw away (see Throwing away (disposing of) OMVOH Prefilled Syringes) if they have been:
- frozen
- microwaved
- warmed with hot water
- left in direct sunlight
- shaken
Keep OMVOH and all medicines out of the reach of children.
Commonly asked questions
Q. The prefilled Syringes are different sizes. Should I take one before the other? A. You can inject the prefilled Syringes in any order. Inject both prefilled Syringes for a full 300 mg dose. Q. What if I see air bubbles in the prefilled Syringes? A. Air bubbles are normal. They will not harm you or affect your dose. Q. What if there is a drop of liquid on the tip of the needle when I remove the needle cap? A. A drop of liquid on the tip of the needle is normal. This will not harm you or affect your dose. Do not touch the needle. Q. What if I cannot push in the plunger? A. If the plunger is stuck or damaged:
Do not continue to use the prefilled Syringe
- Remove the needle from your skin
- Dispose of the prefilled Syringe and get a new one
Q. What if there is a drop of liquid or blood on my skin after my injection? A. This is normal. Press a cotton ball over the injection site. Do not rub the injection site. Q. How can I tell if my injection is complete? A. When your injection is complete:
- The blue plunger rod should show through the body of the prefilled Syringe.
- The gray syringe plunger should be pushed all the way to the needle end of the prefilled Syringe.
Additional Information:
If you have more questions about how to use the OMVOH prefilled Syringes:
- Call your healthcare provider
- Call 1-800-Lilly-Rx (1-800-545-5979)
- Visit www.OMVOH.com

Scan this code to launch www.OMVOH.COM Used OMVOH Prefilled Syringe disposal
Put the used OMVOH prefilled Syringes in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) the OMVOH prefilled Syringes in your household trash.
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container.
There may be state or local laws about how you should throw away needles and syringes.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
Do not recycle your used sharps disposal container.
Read the Medication Guide for OMVOH inside this box to learn more about your medicine.
Manufactured by:
Eli Lilly and Company
Indianapolis, IN 46285, USA
US License Number 1891OMVOHTM is a trademark of Eli Lilly and Company.
Copyright © 2025, Eli Lilly and Company. All rights reserved.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Approved: January 2025
OMV-0001-300MG-PFS-IFU-20250115
-
200 MG PREFILLED PEN INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE Before you use the OMVOH prefilled Pen, read and carefully follow all the step-by-step instructions. Parts of the OMVOH prefilled Pen
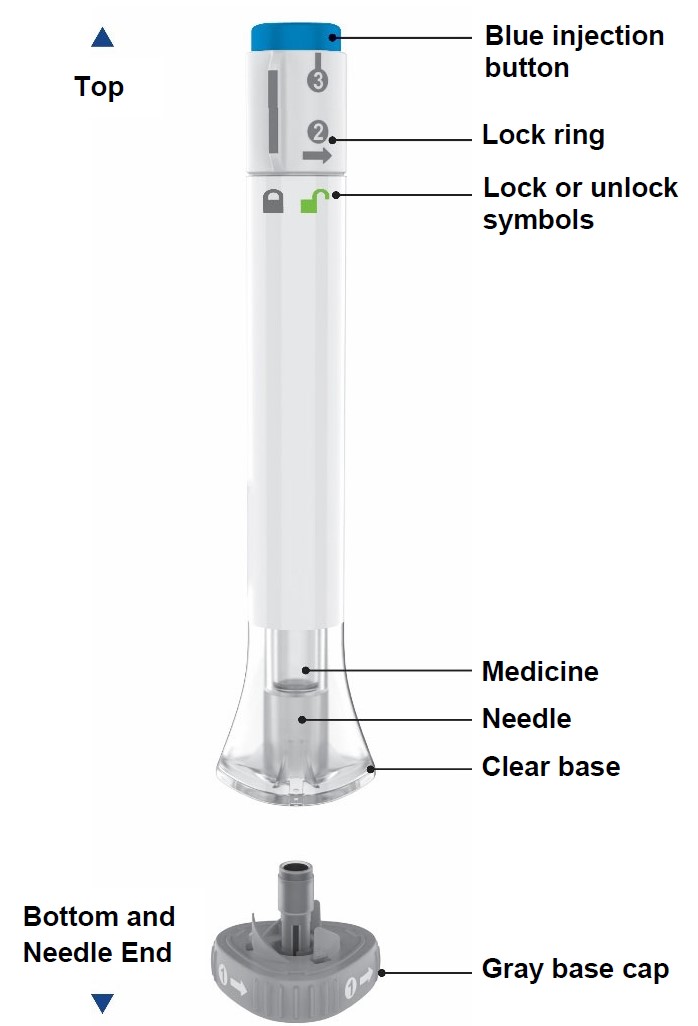
Throwing away (disposing of) OMVOH prefilled Pen
Storing OMVOH Refrigeration
- In the original carton, store your prefilled Pen in the refrigerator between 36°F to 46°F (2°C to 8°C) until the expiration date.
- Do not freeze. Do not use OMVOH if it has been frozen.
Room Temperature
- If needed, your prefilled Pen may be stored at room temperature for up to 2 weeks in the original carton. Do not store above 86°F (30°C).
- When OMVOH has been stored at room temperature, do not return it to the refrigerator.
- Throw away (dispose of) OMVOH if not used within 2 weeks at room temperature.
- Protect your prefilled Pen from light until use.
Do not use the prefilled Pen and throw away (see Throwing away (disposing of) OMVOH prefilled Pen) if it has been:
- frozen
- microwaved
- warmed with hot water
- left in direct sunlight
- shaken
Manufactured by:
Eli Lilly and Company
Indianapolis, IN 46285, USA
US License Number 1891OMVOH™ is a trademark of Eli Lilly and Company.
Copyright © 2025, Eli Lilly and Company. All rights reserved.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Approved: October 2025
The OMVOH prefilled Pen meets the current dose accuracy and functional requirements of ISO 11608-1 and 11608-5.
OMV-0001-200MG-PEN-IFU-20251023
-
200 MG PREFILLED SYRINGE INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE Before you use the OMVOH prefilled Syringe, read and carefully follow all the step-by-step instructions. Parts of the OMVOH prefilled Syringe
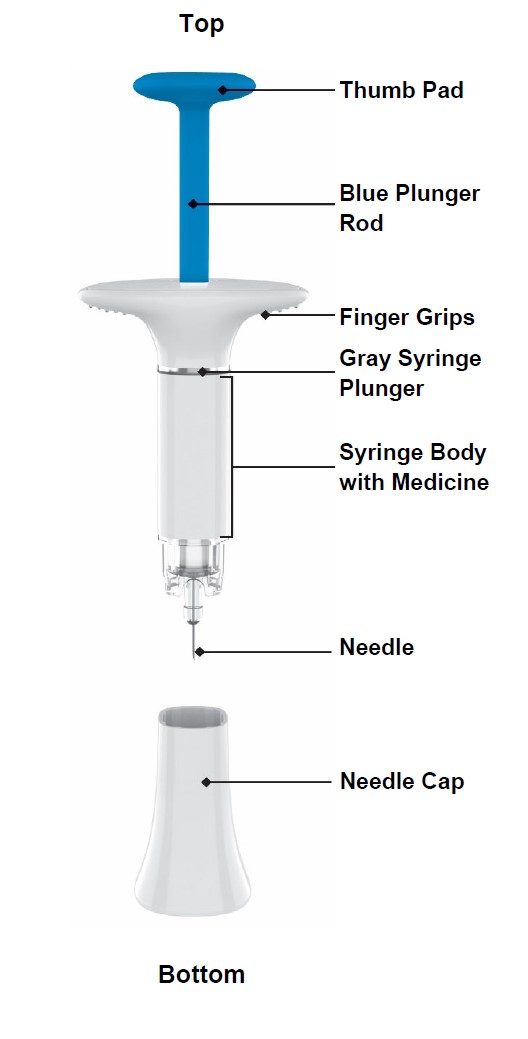
Manufactured by:
Eli Lilly and Company
Indianapolis, IN 46285, USA
US License Number 1891OMVOH is a trademark of Eli Lilly and Company.
Copyright © 2025, Eli Lilly and Company. All rights reserved.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Approved: October 2025
OMV-0001-200MG-PFS-IFU-20251023
-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL – Omvoh 300 mg Vial Cartons
There are two formulations for this product
NDC: 0002-7575-01
15 mL
omvohTM
(mirikizumab-mrkz)
injection
300 mg/15 mL
(20 mg/mL)
For Intravenous Infusion After Dilution
Single-Dose Vial - Discard Unused Portion
Dispense enclosed Medication Guide to each patient.
Rx only
omvoh.com
Lilly
Classic Formulation contains the following inactive ingredients:anhydrous citric acid, polysorbate 80, sodium chloride, sodium citrate, and Water for Injection.
Alternate Formulation contains the following inactive ingredients: anhydrous citric acid, histidine, L-histidine hydrochloride monohydrate, mannitol, polysorbate 80, sodium chloride, sodium citrate, and Water for Injection.
-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL – Omvoh Prefilled Pen 200 mg Dose Carton - Ulcerative Colitis
NDC: 0002-8011-27
omvohTM
(mirikizumab-mrkz)
injection100 mg/mL
For Subcutaneous Use Only
This carton contains a total dose of 200 mg.
Inject both prefilled pens one after the other for a full dose of 200 mg.Note to Pharmacist: The entire carton is to be dispensed as a unit.
2 x 1 mL single-dose prefilled pens
Rx only
Dispense enclosed Medication Guide to each patient.
Lilly
-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL – Omvoh Prefilled Syringe 200 mg Dose Carton - Ulcerative Colitis
NDC: 0002-8870-27
omvohTM
(mirikizumab-mrkz)
injection100 mg/mL
For Subcutaneous Use Only
Note to Pharmacist: The entire carton is to be dispensed as a unit.
This carton contains a total dose of 200 mg.
Inject both prefilled syringes one after the other for a full dose of 200 mg.2 x 1 mL single-dose prefilled syringes
Rx only
Dispense enclosed Medication Guide to each patient.
Lilly
-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL – Omvoh Prefilled Pen 300 mg Dose Carton - Crohns Disease
NDC: 0002-7717-11
omvohTM
(mirikizumab-mrkz)
injection200 mg/2 mL
100 mg/mLFor Subcutaneous Use Only
Note to Pharmacist: The entire carton is to be dispensed as a unit.
This carton contains a total dose of 300 mg.
Inject both prefilled pens one after the other for a full dose of 300 mg.1 x 2 mL single-dose prefilled pen
1 x 1 mL single-dose prefilled penRx only
Dispense enclosed Medication Guide to each patient.
Lilly
-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL – Omvoh Prefilled Syringe 300 mg Dose Carton - Crohns Disease
NDC: 0002-7722-11
omvohTM
(mirikizumab-mrkz)
injection200 mg/2 mL
(100 mg/mL)
100 mg/mLFor Subcutaneous Use Only
Note to Pharmacist: The entire carton is to be dispensed as a unit.
This carton contains a total dose of 300 mg.
Inject both prefilled syringes one after the other for a full dose of 300 mg.1 x 2 mL single-dose prefilled syringe
1 x 1 mL single-dose prefilled syringeRx only
Dispense enclosed Medication Guide to each patient.
Lilly
-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL – Omvoh Prefilled Pen 200 mg Single Injection Dose Carton - Ulcerative Colitis
NDC: 0002-3116-11
omvohTM
(mirikizumab-mrkz)
injection200 mg/2 mL
For Subcutaneous Use Only
This carton contains a total dose of 200 mg.
Only one injection is required for a full dose.1 x 200mg/2mL single dose prefilled pen
Rx only
Dispense enclosed Medication Guide to each patient.
Lilly
-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL – Omvoh Prefilled Syringe 200 mg Single Injection Dose Carton - Ulcerative Colitis
NDC: 0002-1442-11
omvohTM
(mirikizumab-mrkz)
injection200 mg/2 mL
(100 mg/mL)For Subcutaneous Use Only
This carton contains a total dose of 200 mg.
Only one injection is required for a full dose.1 x 200 mg/2 mL single-dose prefilled syringe
Rx only
Dispense enclosed Medication Guide to each patient.
Lilly
-
INGREDIENTS AND APPEARANCE
OMVOH
mirikizumab-mrkz injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0002-7575 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength mirikizumab (UNII: Z7HVY03PHP) (mirikizumab - UNII:Z7HVY03PHP) mirikizumab 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength trisodium citrate dihydrate (UNII: B22547B95K) anhydrous citric acid (UNII: XF417D3PSL) sodium chloride (UNII: 451W47IQ8X) polysorbate 80 (UNII: 6OZP39ZG8H) water (UNII: 059QF0KO0R) Other Ingredients Ingredient Kind Ingredient Name Quantity May contain histidine hydrochloride monohydrate (UNII: X573657P6P) May contain histidine (UNII: 4QD397987E) May contain mannitol (UNII: 3OWL53L36A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0002-7575-01 1 in 1 CARTON 10/26/2023 1 15 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761279 10/26/2023 OMVOH
mirikizumab-mrkz injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0002-8011 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength mirikizumab (UNII: Z7HVY03PHP) (mirikizumab - UNII:Z7HVY03PHP) mirikizumab 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength histidine (UNII: 4QD397987E) 0.1 mg in 1 mL histidine monohydrochloride monohydrate (UNII: X573657P6P) 0.9 mg in 1 mL mannitol (UNII: 3OWL53L36A) 33 mg in 1 mL polysorbate 80 (UNII: 6OZP39ZG8H) 0.3 mg in 1 mL sodium chloride (UNII: 451W47IQ8X) 2.9 mg in 1 mL water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0002-8011-27 2 in 1 CARTON 10/26/2023 1 NDC: 0002-8011-01 1 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC: 0002-8011-61 2 in 1 CARTON 01/16/2024 2 1 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761279 10/26/2023 OMVOH
mirikizumab-mrkz injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0002-8870 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength mirikizumab (UNII: Z7HVY03PHP) (mirikizumab - UNII:Z7HVY03PHP) mirikizumab 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength histidine (UNII: 4QD397987E) 0.1 mg in 1 mL histidine monohydrochloride monohydrate (UNII: X573657P6P) 0.9 mg in 1 mL mannitol (UNII: 3OWL53L36A) 33 mg in 1 mL polysorbate 80 (UNII: 6OZP39ZG8H) 0.3 mg in 1 mL sodium chloride (UNII: 451W47IQ8X) 2.9 mg in 1 mL water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0002-8870-27 2 in 1 CARTON 04/29/2024 1 NDC: 0002-8870-01 1 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761279 04/29/2024 OMVOH
mirikizumab-mrkz kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0002-7717 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0002-7717-11 1 in 1 CARTON; Type 1: Convenience Kit of Co-Package 01/15/2025 2 NDC: 0002-7717-61 1 in 1 CARTON; Type 1: Convenience Kit of Co-Package 01/15/2025 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 SYRINGE 1 mL Part 2 1 SYRINGE 2 mL Part 1 of 2 OMVOH
mirikizumab-mrkz injection, solutionProduct Information Item Code (Source) NDC: 0002-8011 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength mirikizumab (UNII: Z7HVY03PHP) (mirikizumab - UNII:Z7HVY03PHP) mirikizumab 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength histidine (UNII: 4QD397987E) 0.1 mg in 1 mL histidine monohydrochloride monohydrate (UNII: X573657P6P) 0.9 mg in 1 mL mannitol (UNII: 3OWL53L36A) 33 mg in 1 mL polysorbate 80 (UNII: 6OZP39ZG8H) 0.3 mg in 1 mL sodium chloride (UNII: 451W47IQ8X) 2.9 mg in 1 mL water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0002-8011-01 1 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 1 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761279 01/15/2025 Part 2 of 2 OMVOH
mirikizumab-mrkz injection, solutionProduct Information Item Code (Source) NDC: 0002-3116 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength mirikizumab (UNII: Z7HVY03PHP) (mirikizumab - UNII:Z7HVY03PHP) mirikizumab 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength histidine (UNII: 4QD397987E) 0.1 mg in 1 mL histidine monohydrochloride monohydrate (UNII: X573657P6P) 0.9 mg in 1 mL mannitol (UNII: 3OWL53L36A) 33 mg in 1 mL polysorbate 80 (UNII: 6OZP39ZG8H) 0.3 mg in 1 mL sodium chloride (UNII: 451W47IQ8X) 2.9 mg in 1 mL water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0002-3116-01 2 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 2 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761279 01/15/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761279 01/15/2025 OMVOH
mirikizumab-mrkz kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0002-7722 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0002-7722-11 1 in 1 CARTON; Type 1: Convenience Kit of Co-Package 01/15/2025 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 SYRINGE 1 mL Part 2 1 SYRINGE 2 mL Part 1 of 2 OMVOH
mirikizumab-mrkz injection, solutionProduct Information Item Code (Source) NDC: 0002-8870 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength mirikizumab (UNII: Z7HVY03PHP) (mirikizumab - UNII:Z7HVY03PHP) mirikizumab 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength histidine (UNII: 4QD397987E) 0.1 mg in 1 mL histidine monohydrochloride monohydrate (UNII: X573657P6P) 0.9 mg in 1 mL mannitol (UNII: 3OWL53L36A) 33 mg in 1 mL polysorbate 80 (UNII: 6OZP39ZG8H) 0.3 mg in 1 mL sodium chloride (UNII: 451W47IQ8X) 2.9 mg in 1 mL water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0002-8870-01 1 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761279 01/15/2025 Part 2 of 2 OMVOH
mirikizumab-mrkz injection, solutionProduct Information Item Code (Source) NDC: 0002-1442 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength mirikizumab (UNII: Z7HVY03PHP) (mirikizumab - UNII:Z7HVY03PHP) mirikizumab 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength histidine (UNII: 4QD397987E) 0.1 mg in 1 mL histidine monohydrochloride monohydrate (UNII: X573657P6P) 0.9 mg in 1 mL mannitol (UNII: 3OWL53L36A) 33 mg in 1 mL polysorbate 80 (UNII: 6OZP39ZG8H) 0.3 mg in 1 mL sodium chloride (UNII: 451W47IQ8X) 2.9 mg in 1 mL water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0002-1442-01 2 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761279 01/15/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761279 01/15/2025 OMVOH
mirikizumab-mrkz injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0002-3116 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength mirikizumab (UNII: Z7HVY03PHP) (mirikizumab - UNII:Z7HVY03PHP) mirikizumab 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength histidine (UNII: 4QD397987E) 0.1 mg in 1 mL histidine monohydrochloride monohydrate (UNII: X573657P6P) 0.9 mg in 1 mL mannitol (UNII: 3OWL53L36A) 33 mg in 1 mL polysorbate 80 (UNII: 6OZP39ZG8H) 0.3 mg in 1 mL sodium chloride (UNII: 451W47IQ8X) 2.9 mg in 1 mL water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0002-3116-11 1 in 1 CARTON 10/23/2025 1 NDC: 0002-3116-01 2 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC: 0002-3116-61 1 in 1 CARTON 11/06/2025 2 2 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761279 10/23/2025 OMVOH
mirikizumab-mrkz injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0002-1442 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength mirikizumab (UNII: Z7HVY03PHP) (mirikizumab - UNII:Z7HVY03PHP) mirikizumab 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength histidine (UNII: 4QD397987E) 0.1 mg in 1 mL histidine monohydrochloride monohydrate (UNII: X573657P6P) 0.9 mg in 1 mL mannitol (UNII: 3OWL53L36A) 33 mg in 1 mL polysorbate 80 (UNII: 6OZP39ZG8H) 0.3 mg in 1 mL sodium chloride (UNII: 451W47IQ8X) 2.9 mg in 1 mL water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0002-1442-11 1 in 1 CARTON 10/23/2025 1 NDC: 0002-1442-01 2 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761279 10/23/2025 Labeler - Eli Lilly and Company (006421325)
Trademark Results [Omvoh]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 OMVOH 97897913 not registered Live/Pending |
Eli Lilly and Company 2023-04-20 |
 OMVOH 97041869 not registered Live/Pending |
Eli Lilly and Company 2021-09-23 |
 OMVOH 88729726 not registered Live/Pending |
Eli Lilly and Company 2019-12-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.