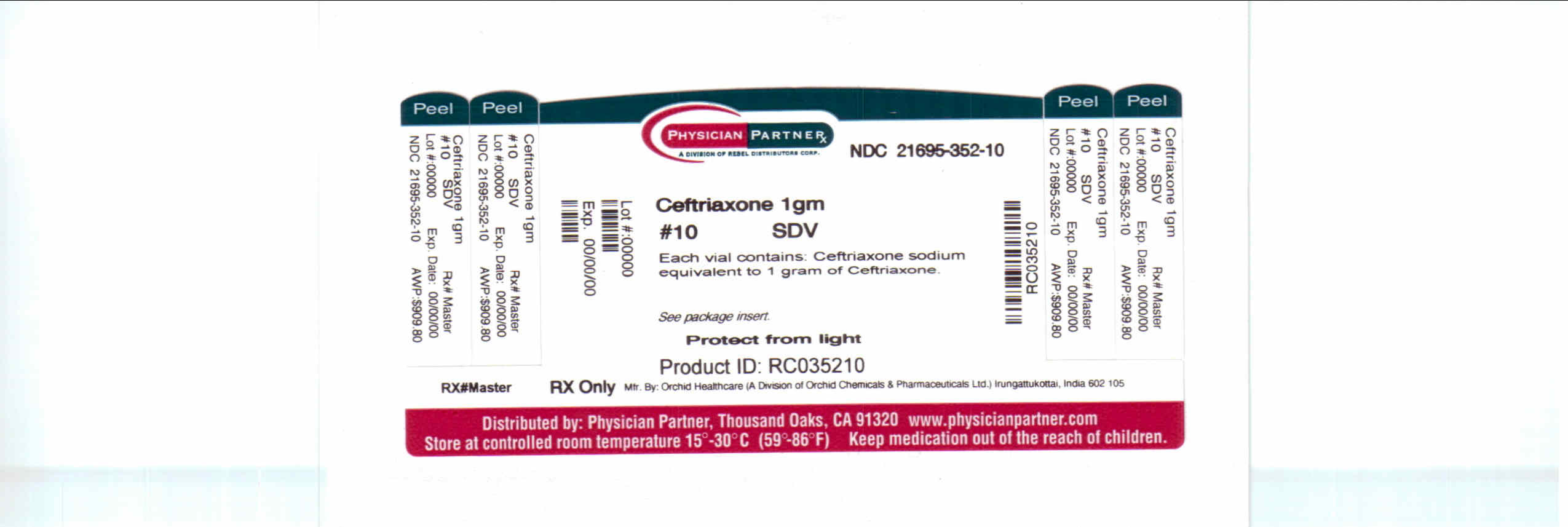
CEFTRIAXONE SODIUM injection, powder, for solution
Ceftriaxone Sodium by
Drug Labeling and Warnings
Ceftriaxone Sodium by is a Prescription medication manufactured, distributed, or labeled by Rebel Distributors Corp. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- To reduce the development of drug-resistant bacteria and maintain the effectiveness of ceftriaxone for injection and other antibacterial drugs, ceftriaxone for injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
-
DESCRIPTION
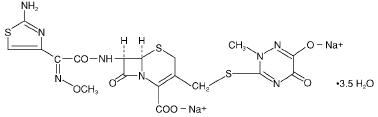
Ceftriaxone sodium is a sterile, semisynthetic, broad-spectrum cephalosporin antibiotic for intravenous or intramuscular administration. Ceftriaxone sodium is (6R, 7R)-7-[2-(2-Amino-4-thiazolyl) glyoxylamido]-8-oxo-3- [[(1,2,5,6-tetrahydro-2-methyl-5,6-dioxo-as-triazin-3-yl)thio]methyl]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 72-(Z)-(O-methyloxime), disodium salt, sesquaterhydrate.
The chemical formula of ceftriaxone sodium is C18H16N8Na2O7S33.5H2O. It has a calculated molecular weight of 661.59 and the following structural formula:
Ceftriaxone sodium is a white to yellowish-orange crystalline powder which is readily soluble in water, sparingly soluble in methanol and very slightly soluble in ethanol. The pH of a 1% aqueous solution is approximately 6.7. The color of ceftriaxone sodium solutions ranges from light yellow to amber, depending on the length of storage, concentration and diluent used.
Ceftriaxone sodium contains approximately 83 mg (3.6 mEq) of sodium per gram of ceftriaxone activity.
-
CLINICAL PHARMACOLOGY
Average plasma concentrations of ceftriaxone following a single 30-minute intravenous (IV) infusion of a 0.5, 1 or 2 gm dose and intramuscular (IM) administration of a single 0.5 (250 mg/mL or 350 mg/mL concentrations) or 1 gm dose in healthy subjects are presented in Table 1.
TABLE 1 Ceftriaxone Plasma Concentrations After Single Dose Administration Dose/Route Average Plasma Concentrations (mcg/mL) - * IV doses were infused at a constant rate over 30 minutes.
- † Not Determined
0.5 hr 1 hr 2 hr 4 hr 6 hr 8 hr 12 hr 16 hr 24 hr 0.5 gm IV* 82 59 48 37 29 23 15 10 5 0.5 gm IM 250 mg/mL 22 33 38 35 30 26 16 ND† 5 0.5 gm IM 350 mg/mL 20 32 38 34 31 24 16 ND † 5 1 gm IV * 151 111 88 67 53 43 28 18 9 1 gm IM 40 68 76 68 56 44 29 ND † ND † 2 gm IV * 257 192 154 117 89 74 46 31 15 Ceftriaxone was completely absorbed following IM administration with mean maximum plasma concentrations occurring between 2 and 3 hours post-dose. Multiple IV or IM doses ranging from 0.5 to 2 gm at 12- to 24-hour intervals resulted in 15% to 36% accumulation of ceftriaxone above single dose values.
Ceftriaxone concentrations in urine are shown in Table 2.
TABLE 2 Urinary Concentrations of Ceftriaxone After Single Dose Administration Dose/Route Average Urinary Concentrations (mcg/mL) 0-2 hr 2-4 hr 4-8 hr 8-12 hr 12-24 hr 24-48 hr 0.5 gm IV 526 366 142 87 70 15 0.5 gm IM 115 425 308 127 96 28 1 gm IV 995 855 293 147 132 32 1 gm IM 504 628 418 237 ND † ND † 2 gm IV 2692 1976 757 274 198 40 Thirty-three percent to 67% of a ceftriaxone dose was excreted in the urine as unchanged drug and the remainder was secreted in the bile and ultimately found in the feces as microbiologically inactive compounds. After a 1 gm IV dose, average concentrations of ceftriaxone, determined from 1 to 3 hours after dosing, were 581 mcg/mL in the gallbladder bile, 788 mcg/mL in the common duct bile, 898 mcg/mL in the cystic duct bile, 78.2 mcg/gm in the gallbladder wall and 62.1 mcg/mL in the concurrent plasma.
Over a 0.15 to 3 gm dose range in healthy adult subjects, the values of elimination half-life ranged from 5.8 to 8.7 hours; apparent volume of distribution from 5.78 to 13.5 L; plasma clearance from 0.58 to 1.45 L/hour; and renal clearance from 0.32 to 0.73 L/hour. Ceftriaxone is reversibly bound to human plasma proteins, and the binding decreased from a value of 95% bound at plasma concentrations of < 25 mcg/mL to a value of 85% bound at 300 mcg/mL. Ceftriaxone crosses the blood placenta barrier.
The average values of maximum plasma concentration, elimination half-life, plasma clearance and volume of distribution after a 50 mg/kg IV dose and after a 75 mg/kg IV dose in pediatric patients suffering from bacterial meningitis are shown in Table 3. Ceftriaxone penetrated the inflamed meninges of infants and pediatric patients; CSF concentrations after a 50 mg/kg IV dose and after a 75 mg/kg IV dose are also shown in Table 3.
TABLE 3 Average Pharmacokinetic Parameters of Ceftriaxone in Pediatric Patients With Meningitis 50 mg/kg IV 75 mg/kg IV Maximum Plasma Concentrations (mcg/mL) 216 275 Elimination Half-life (hr) 4.6 4.3 Plasma Clearance (mL/hr/kg) 49 60 Volume of Distribution (mL/kg) 338 373 CSF Concentration-inflamed meninges (mcg/mL) 5.6 6.4 Range (mcg/mL) 1.3 – 18.5 1.3-44 Time after dose (hr) 3.7 (± 1.6) 3.3 (± 1.4) Compared to that in healthy adult subjects, the pharmacokinetics of ceftriaxone were only minimally altered in elderly subjects and in patients with renal impairment or hepatic dysfunction (Table 4); therefore, dosage adjustments are not necessary for these patients with ceftriaxone dosages up to 2 gm per day. Ceftriaxone was not removed to any significant extent from the plasma by hemodialysis; in six of 26 dialysis patients, the elimination rate of ceftriaxone was markedly reduced.
TABLE 4 Average Pharmacokinetic Parameters of Ceftriaxone in Humans Subject Group Elimination
Half-Life (hr)Plasma
Clearance (L/hr)Volume of Distribution (L) - * Creatinine clearance.
Healthy Subjects 5.8 – 8.7 0.58 – 1.45 5.8 – 13.5 Elderly Subjects (mean age, 70.5 yr) 8.9 0.83 10.7 Patients With Renal Impairment Hemodialysis Patients (0-5 mL/min)* 14.7 0.65 13.7 Severe (5-15 mL/min) 15.7 0.56 12.5 Moderate (16-30 mL/min) 11.4 0.72 11.8 Mild (31-60 mL/min) 12.4 0.7 13.3 Patients With Liver Disease 8.8 1.1 13.6 The elimination of ceftriaxone is not altered when ceftriaxone is co-administered with probenecid.
Pharmacokinetics in the Middle Ear Fluid
In one study, total ceftriaxone concentrations (bound and unbound) were measured in middle ear fluid obtained during the insertion of tympanostomy tubes in 42 pediatric patients with otitis media. Sampling times were from 1 to 50 hours after a single intramuscular injection of 50 mg/kg of ceftriaxone. Mean (± SD) ceftriaxone levels in the middle ear reached a peak of 35 (± 12) mcg/mL at 24 hours, and remained at 19 (± 7) mcg/mL at 48 hours. Based on middle ear fluid ceftriaxone concentrations in the 23 to 25 hour and the 46 to 50 hour sampling time intervals, a half-life of 25 hours was calculated. Ceftriaxone is highly bound to plasma proteins. The extent of binding to proteins in the middle ear fluid is unknown.
Interaction with Calcium: Two in vitro studies, one using adult plasma and the other neonatal plasma from umbilical cord blood have been carried out to assess interaction of ceftriaxone and calcium. Ceftriaxone concentrations up to 1 mM (in excess of concentrations achieved in vivo following administration of 2 grams ceftriaxone infused over 30 minutes) were used in combination with calcium concentrations up to 12 mM (48 mg/dL). Recovery of ceftriaxone from plasma was reduced with calcium concentrations of 6 mM (24 mg/dL) or higher in adult plasma or 4 mM (16 mg/dL) or higher in neonatal plasma. This may be reflective of ceftriaxone-calcium precipitation.
Microbiology
The bactericidal activity of ceftriaxone results from inhibition of cell wall synthesis. Ceftriaxone has a high degree of stability in the presence of beta-lactamases, both penicillinases and cephalosporinases, of gram-negative and gram-positive bacteria.
In an in vitro study antagonistic effects have been observed with the combination of chloramphenicol and ceftriaxone.
Ceftriaxone has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections described in the INDICATIONS AND USAGE section.
Aerobic gram-negative microorganisms
Acinetobacter calcoaceticus
Enterobacter aerogenes
Enterobacter cloacae
Escherichia coli
Haemophilus influenzae (including ampicillin-resistant and beta-lactamase producing strains)
Haemophilus parainfluenzae
Klebsiella oxytoca
Klebsiella pneumoniae
Moraxella catarrhalis (including beta-lactamase producing strains)
Morganella morganii
Neisseria gonorrhoeae (including penicillinase- and nonpenicillinase-producing strains)
Neisseria meningitidis
Proteus mirabilis
Proteus vulgaris
Serratia marcescens
Ceftriaxone is also active against many strains of Pseudomonas aeruginosa.
NOTE: Many strains of the above organisms that are resistant to multiple antibiotics, e.g., penicillins, cephalosporins, and aminoglycosides, are susceptible to ceftriaxone.
Aerobic gram-positive microorganisms
Staphylococcus aureus (including penicillinase-producing strains)
Staphylococcus epidermidis
Streptococcus pneumoniae
Streptococcus pyogenes
Viridans group streptococci
NOTE: Methicillin-resistant staphylococci are resistant to cephalosporins, including ceftriaxone. Most strains of Group D streptococci and enterococci, e.g., Enterococcus (Streptococcus) faecalis, are resistant.
Anaerobic microorganisms
Bacteroides fragilis
Clostridium species
Peptostreptococcus species
NOTE: Most strains of Clostridium difficile are resistant.
The following in vitro data are available, but their clinical significance is unknown. Ceftriaxone exhibits in vitro minimal inhibitory concentrations (MICs) of ≤ 8 mcg/mL or less against most strains of the following microorganisms, however, the safety and effectiveness of ceftriaxone in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled clinical trials.
Aerobic gram-negative microorganisms
Citrobacter diversus
Citrobacter freundii
Providencia species (including Providencia rettgeri)
Salmonella species (including Salmonella typhi)
Shigella species
Anaerobic microorganisms
Prevotella (Bacteroides) bivius
Porphyromonas (Bacteroides) melaninogenicus
Susceptibility Tests
Dilution Techniques
Quantitative methods are used to determine antimicrobial minimal inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized procedure1. Standardized procedures are based on a dilution method (broth or agar) or equivalent with standardized inoculum concentrations and standardized concentrations of ceftriaxone powder. The MIC values should be interpreted according to the following criteria2 for aerobic organisms other than Haemophilus spp, Neisseria gonorrhoeae, and Streptococcus spp, including Streptococcus pneumoniae:
MIC (mcg/mL) Interpretation ≤ 8 (S) Susceptible 16-32 (I) Intermediate ≥ 64 (R) Resistant The following interpretive criteria3 should be used when testing Haemophilus species using Haemophilus Test Media (HTM).
MIC (mcg/mL) Interpretation ≤ 2 (S) Susceptible The absence of resistant strains precludes defining any categories other than "Susceptible". Strains yielding results suggestive of a "Nonsusceptible" category should be submitted to a reference laboratory for further testing.
The following interpretive criteria4 should be used when testing Neisseria gonorrhoeae when using GC agar base and 1% defined growth supplement.
MIC (mcg/mL) Interpretation ≤ 0.25 (S) Susceptible The absence of resistant strains precludes defining any categories other than "Susceptible". Strains yielding results suggestive of a "Nonsusceptible" category should be submitted to a reference laboratory for further testing.
The following interpretive criteria5 should be used when testing Streptococcus spp including Streptococcus pneumoniae using cation-adjusted Mueller-Hinton broth with 2 to 5% lysed horse blood.
MIC (mcg/mL) Interpretation ≤ 0.5 (S) Susceptible 1 (I) Intermediate ≥ 2 (R) Resistant A report of "Susceptible" indicates that the pathogen is likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable. A report of “Intermediate” indicates that the results should be considered equivocal, and if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of the drug can be used. This category also provides a buffer zone which prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of "Resistant" indicates that the pathogen is not likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable; other therapy should be selected.
Standardized susceptibility test procedures require the use of laboratory control microorganisms to control the technical aspects of the laboratory procedures. Standardized ceftriaxone powder should provide the following MIC values:6
Microorganism ATCC® # MIC (mcg/mL) - * A bimodal distribution of MICs results at the extremes of the acceptable range should be suspect and control validity should be verified with data from other control strains.
Escherichia coli 25922 0.03-0.12 Staphylococcus aureus 29213 1- 8* Pseudomonas aeruginosa 27853 8-32 Haemophilus influenzae 49247 0.06 - 0.25 Neisseria gonorrhoeae 49226 0.004-0.015 Streptococcus pneumoniae 49619 0.03 -0.12
- 1
National Committee for Clinical Laboratory Standards, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard-Fifth Edition. NCCLS document M7-A5 (ISBN 1-56238-309-9). NCCLS, Wayne, PA 19087-1898, 2000.
- 2
National Committee for Clinical Laboratory Standards, Supplemental Tables. NCCLS document M100-S10 (M7) (ISBN 1 - 56238-309-9). NCCLS, Wayne, PA 19087-1898, 2000.
- 3
National Committee for Clinical Laboratory Standards, Supplemental Tables. NCCLS document M100-S10 (M7) (ISBN 1 - 56238-309-9). NCCLS, Wayne, PA 19087-1898, 2000.
- 4
National Committee for Clinical Laboratory Standards, Supplemental Tables. NCCLS document M100-S10 (M7) (ISBN 1 - 56238-309-9). NCCLS, Wayne, PA 19087-1898, 2000.
- 5
National Committee for Clinical Laboratory Standards, Supplemental Tables. NCCLS document M100-S10 (M7) (ISBN 1 - 56238-309-9). NCCLS, Wayne, PA 19087-1898, 2000.
- 6
National Committee for Clinical Laboratory Standards, Supplemental Tables. NCCLS document M100-S10 (M7) (ISBN 1 - 56238-309-9). NCCLS, Wayne, PA 19087-1898, 2000.
Diffusion Techniques
Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure7 requires the use of standardized inoculum concentrations. This procedure uses paper discs impregnated with 30 mcg of ceftriaxone to test the susceptibility of microorganisms to ceftriaxone.
Reports from the laboratory providing results of the standard single-disc susceptibility test with a 30 mcg ceftriaxone disc should be interpreted according to the following criteria for aerobic organisms other than Haemophilus spp, Neisseria gonorrhoeae, and Streptoccocus spp:
Zone diameter (mm) Interpretation ≥ 21 (S) Susceptible 14-20 (I) Intermediate ≤ 13 (R) Resistant The following interpretive criteria8 should be used when testing Haemophilus species when using Haemophilus Test Media (HTM).
Zone diameter (mm) Interpretation ≥ 26 (S) Susceptible The absence of resistant strains precludes defining any categories other than "Susceptible". Strains yielding results suggestive of a "Nonsusceptible" category should be submitted to a reference laboratory for further testing.
The following interpretive criteria9 should be used when testing Neisseria gonorrhoeae when using GC agar base and 1 % defined growth supplement.
Zone diameter (mm) Interpretation ≥ 35 (S) Susceptible The absence of resistant strains precludes defining any categories other than "Susceptible". Strains yielding results suggestive of a "Nonsusceptible" category should be submitted to a reference laboratory for further testing.
The following interpretive criteria10 should be used when testing Streptococcus spp other than Streptococcus pneumoniae when using Mueller-Hinton agar supplemented with 5% sheep blood incubated in 5% CO2.
Zone diameter (mm) Interpretation ≥ 27 (S) Susceptible 25-26 (I) Intermediate ≤ 24 (R) Resistant Interpretation should be as stated above for results using dilution techniques. Interpretation involves correlation of the diameter obtained in the disc test with the MIC for ceftriaxone.
Disc diffusion interpretive criteria for ceftriaxone discs against Streptococcus pneumoniae are not available, however, isolates of pneumococci with oxacillin zone diameters of > 20 mm are susceptible (MIC ≤ 0.06 mcg/mL) to penicillin and can be considered susceptible to ceftriaxone. Streptococcus pneumoniae isolates should not be reported as penicillin (ceftriaxone) resistant or intermediate based solely on an oxacillin zone diameter of ≤ 19 mm. The ceftriaxone MIC should be determined for those isolates with oxacillin zone diameters ≤ 19 mm.
As with standardized dilution techniques, diffusion methods require the use of laboratory control microorganisms that are used to control the technical aspects of the laboratory procedures. For the diffusion technique, the 30 mcg ceftriaxone disc should provide the following zone diameters in these laboratory test quality control strains:11
Microorganism ATCC® # Zone Diameter Ranges (mm) Escherichia coli 25922 29-35 Staphylococcus aureus 25923 22-28 Pseudomonas aeruginosa 27853 17-23 Haemophilus influenzae 49247 31-39 Neisseria gonorrhoeae 49226 39-51 Streptococcus pneumoniae 49619 30-35
- 7
National Committee for Clinical Laboratory Standards, Performance Standards for Antimicrobial Disc Susceptibility Tests; Approved Standard - Seventh Edition. NCCLS document M2-A7 (ISBN 1-56238-393-0). NCCLS, Wayne, PA 19087-1898, 2000.
- 8
National Committee for Clinical Laboratory Standards, Performance Standards for Antimicrobial Disc Susceptibility Tests; Approved Standard - Seventh Edition. NCCLS document M2-A7 (ISBN 1-56238-393-0). NCCLS, Wayne, PA 19087-1898, 2000.
- 9
National Committee for Clinical Laboratory Standards, Performance Standards for Antimicrobial Disc Susceptibility Tests; Approved Standard - Seventh Edition. NCCLS document M2-A7 (ISBN 1-56238-393-0). NCCLS, Wayne, PA 19087-1898, 2000.
- 10
National Committee for Clinical Laboratory Standards, Performance Standards for Antimicrobial Disc Susceptibility Tests; Approved Standard - Seventh Edition. NCCLS document M2-A7 (ISBN 1-56238-393-0). NCCLS, Wayne, PA 19087-1898, 2000.
- 11
National Committee for Clinical Laboratory Standards, Performance Standards for Antimicrobial Disc Susceptibility Tests; Approved Standard - Seventh Edition. NCCLS document M2-A7 (ISBN 1-56238-393-0). NCCLS, Wayne, PA 19087-1898, 2000.
Anaerobic Techniques
For anaerobic bacteria, the susceptibility to ceftriaxone as MICs can be determined by standardized test methods.12 The MIC values obtained should be interpreted according to the following criteria:
MIC (mcg/mL) Interpretation ≤ 16 (S) Susceptible 32 (I) Intermediate ≥ 64 (R) Resistant As with other susceptibility techniques, the use of laboratory control microorganism is required to control the technical aspects of the laboratory standardized procedures. Standardized ceftriaxone powder should provide the following MIC values for the indicated standardized anaerobic dilution13 testing method:
Method Microorganism ATCC ® # MIC (mcg/mL) Agar Bacteroides fragilis 25285 32 -128 Bacteroides thetaiotaomicron 29741 64 -256 Broth Bacteroides thetaiotaomicron 29741 32 -128 ATCC® is a registered trademark of the American Type Culture Collection.
- 12
National Committee for Clinical Laboratory Standards, Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard-Fourth Edition. NCCLS document M11-A4 (ISBN 1-56238-210-1). NCCLS, Wayne, PA 19087-1898, 1997.
- 13
National Committee for Clinical Laboratory Standards, Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard-Fourth Edition. NCCLS document M11-A4 (ISBN 1-56238-210-1). NCCLS, Wayne, PA 19087-1898, 1997.
-
INDICATIONS & USAGE
Before instituting treatment with ceftriaxone, appropriate specimens should be obtained for isolation of the causative organism and for determination of its susceptibility to the drug. Therapy may be instituted prior to obtaining results of susceptibility testing.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of ceftriaxone for injection and other antibacterial drugs, ceftriaxone for injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Ceftriaxone sodium is indicated for the treatment of the following infections when caused by susceptible organisms:
Lower Respiratory Tract Infections caused by Streptococcus pneumoniae, Staphylococcus aureus, Haemophilus influenzae, Haemophilus parainfluenzae, Klebsiella pneumoniae, Escherichia coli, Enterobacter aerogenes, Proteus mirabilis or Serratia marcescens.
Acute Bacterial Otitis Media caused by Streptococcus pneumoniae, Haemophilus influenzae (including beta-lactamase producing strains) or Moraxella catarrhalis (including beta-lactamase producing strains).
NOTE: In one study lower clinical cure rates were observed with a single dose of ceftriaxone sodium compared to 10 days of oral therapy. In a second study comparable cure rates were observed between single dose ceftriaxone sodium and the comparator. The potentially lower clinical cure rate of ceftriaxone should be balanced against the potential advantages of parenteral therapy (see CLINICAL STUDIES).
Skin And Skin Structure Infections caused by Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pyogenes, Viridans group streptococci, Escherichia coli, Enterobacter cloacae, Klebsiella oxytoca, Klebsiella pneumoniae, Proteus mirabilis, Morganella morganii14, Pseudomonas aeruginosa, Serratia marcescens, Acinetobacter calcoaceticus, Bacteroides fragilis 15 or Peptostreptococcus species.
Urinary Tract Infections (complicated and uncomplicated) caused by Escherichia coli, Proteus mirabilis, Proteus vulgaris, Morganella morganii or Klebsiella pneumoniae.
Uncomplicated Gonorrhea (cervical/urethral and rectal) caused by Neisseria gonorrhoeae, including both penicillinase- and nonpenicillinase-producing strains, and pharyngeal gonorrhea caused by nonpenicillinase-producing strains of Neisseria gonorrhoeae.
Pelvic Inflammatory Disease caused by Neisseria gonorrhoeae. Ceftriaxone sodium, like other cephalosporins, has no activity against Chlamydia trachomatis. Therefore, when cephalosporins are used in the treatment of patients with pelvic inflammatory disease and Chlamydia trachomatis is one of the suspected pathogens, appropriate antichlamydial coverage should be added.
Bacterial Septicemia caused by Staphylococcus aureus, Streptococcus pneumoniae, Escherichia coli, Haemophilus influenzae or Klebsiella pneumoniae.
Bone And Joint Infections caused by Staphylococcus aureus, Streptococcus pneumoniae, Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae or Enterobacter species.
Intra-Abdominal Infections caused by Escherichia coli, Klebsiella pneumoniae, Bacteroides fragilis, Clostridium species (Note: most strains of Clostridium difficile are resistant) or Peptostreptococcus species.
Meningitis caused by Haemophilus influenzae, Neisseria meningitidis or Streptococcus pneumoniae. Ceftriaxone has also been used successfully in a limited number of cases of meningitis and shunt infection caused by Staphylococcus epidermidis 16 and Escherichia coli 17.
Surgical Prophylaxis: The preoperative administration of a single 1 gm dose of ceftriaxone sodium may reduce the incidence of postoperative infections in patients undergoing surgical procedures classified as contaminated or potentially contaminated (e.g., vaginal or abdominal hysterectomy or cholecystectomy for chronic calculous cholecystitis in high-risk patients, such as those over 70 years of age, with acute cholecystitis not requiring therapeutic antimicrobials, obstructive jaundice or common duct bile stones) and in surgical patients for whom infection at the operative site would present serious risk (e.g., during coronary artery bypass surgery). Although ceftriaxone has been shown to have been as effective as cefazolin in the prevention of infection following coronary artery bypass surgery, no placebo-controlled trials have been conducted to evaluate any cephalosporin antibiotic in the prevention of infection following coronary artery bypass surgery.
When administered prior to surgical procedures for which it is indicated, a single 1 gm dose of ceftriaxone sodium provides protection from most infections due to susceptible organisms throughout the course of the procedure.
- 14
Efficacy for this organism in this organ system was studied in fewer than ten infections.
- 15
Efficacy for this organism in this organ system was studied in fewer than ten infections.
- 16
Efficacy for this organism in this organ system was studied in fewer than ten infections.
- 17
Efficacy for this organism in this organ system was studied in fewer than ten infections.
- 14
-
CONTRAINDICATIONS
Ceftriaxone is contraindicated in patients with known allergy to the cephalosporin class of antibiotics.
Nonates ( ≤28 days)
Hyperbilirubinemic neonates, especially prematures, should not be treated with ceftriaxone. In vitro studies have shown that ceftriaxone can displace bilirubin from its binding to serum albumin, leading to a possible risk of bilirubin encephalopathy in these patients.
Ceftriaxone is contraindicated in neonates if they require (or are expected to require) treatment with calcium-containing IV solutions, including continuous calcium-containing infusions such as parenteral nutrition because of the risk of precipitation of ceftriaxone-calcium (see CLINICAL PHARMACOLOGY, WARNINGS and DOSAGE AND ADMINISTRATION).
A small number of cases of fatal outcomes in which a crystalline material was observed in the lungs and kidneys at autopsy have been reported in neonates receiving ceftriaxone and calcium-containing fluids. In some of these cases, the same intravenous infusion line was used for both ceftriaxone and calcium-containing fluids and in some a precipitate was observed in the intravenous infusion line. At least one fatality has been reported in a neonate in whom ceftriaxone and calcium-containing fluids were administered at different time points via different intravenous lines; no crystalline material was observed at autopsy in this neonate. There have been no similar reports in patients other than neonates.
-
WARNINGS
Hypersensitivity
BEFORE THERAPY WITH CEFTRIAXONE IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEPHALOSPORINS, PENICILLINS OR OTHER DRUGS. THIS PRODUCT SHOULD BE GIVEN CAUTIOUSLY TO PENICILLIN-SENSITIVE PATIENTS. ANTIBIOTICS SHOULD BE ADMINISTERED WITH CAUTION TO ANY PATIENT WHO HAS DEMONSTRATED SOME FORM OF ALLERGY, PARTICULARLY TO DRUGS. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE THE USE OF SUBCUTANEOUS EPINEPHRINE AND OTHER EMERGENCY MEASURES.
As with other cephalosporins, anaphylactic reactions with fatal outcome have been reported, even if a patient is not known to be allergic or previously exposed.
Interaction with Calcium-Containing Products
Do not use diluents containing calcium, such as Ringer’s solution or Hartmann’s solution, to reconstitute ceftriaxone vials or to further dilute a reconstituted vial for IV administration because a precipitate can form. Precipitation of ceftriaxonecalcium can also occur when ceftriaxone is mixed with calcium-containing solutions in the same IV administration line. Ceftriaxone must not be administered simultaneously with calcium-containing IV solutions, including continuous calcium-containing infusions such as parenteral nutrition via a Y-site. However, in patients other than neonates, ceftriaxone and calcium-containing solutions may be administered sequentially of one another if the infusion lines are thoroughly flushed between infusions with a compatible fluid. In vitro studies using adult and neonatal plasma from umbilical cord blood demonstrated that neonates have an increased risk of precipitation of ceftriaxone-calcium (see CLINICAL PHARMACOLOGY, CONTRAINDICATIONS and DOSAGE AND ADMINISTRATION).
Clostridium difficile
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including ceftriaxone, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Hemolytic Anemia
An immune mediated hemolytic anemia has been observed in patients receiving cephalosporin class antibacterials including ceftriaxone. Severe cases of hemolytic anemia, including fatalities, have been reported during treatment in both adults and children. If a patient develops anemia while on ceftriaxone, the diagnosis of a cephalosporin associated anemia should be considered and ceftriaxone stopped until the etiology is determined.
-
PRECAUTIONS
General
Prescribing ceftriaxone for injection in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Although transient elevations of BUN and serum creatinine have been observed, at the recommended dosages, the nephrotoxic potential of ceftriaxone is similar to that of other cephalosporins.
Ceftriaxone is excreted via both biliary and renal excretion (see CLINICAL PHARMACOLOGY). Therefore, patients with renal failure normally require no adjustment in dosage when usual doses of ceftriaxone are administered.
Dosage adjustments should not be necessary in patients with hepatic dysfunction; however, in patients with both hepatic dysfunction and significant renal disease, caution should be exercised and the ceftriaxone dosage should not exceed 2 gm daily.
Alterations in prothrombin times have occurred rarely in patients treated with ceftriaxone. Patients with impaired vitamin K synthesis or low vitamin K stores (e.g., chronic hepatic disease and malnutrition) may require monitoring of prothrombin time during ceftriaxone treatment. Vitamin K administration (10 mg weekly) may be necessary if the prothrombin time is prolonged before or during therapy.
Prolonged use of ceftriaxone may result in overgrowth of nonsusceptible organisms. Careful observation of the patient is essential. If superinfection occurs during therapy, appropriate measures should be taken.
Ceftriaxone should be prescribed with caution in individuals with a history of gastrointestinal disease, especially colitis.
There have been reports of sonographic abnormalities in the gallbladder of patients treated with ceftriaxone; some of these patients also had symptoms of gallbladder disease. These abnormalities appear on sonography as an echo without acoustical shadowing suggesting sludge or as an echo with acoustical shadowing which may be misinterpreted as gallstones. The chemical nature of the sonographically detected material has been determined to be predominantly a ceftriaxone-calcium salt. The condition appears to be transient and reversible upon discontinuation of ceftriaxone and institution of conservative management. Therefore, ceftriaxone should be discontinued in patients who develop signs and symptoms suggestive of gallbladder disease and/or the sonographic findings described above.
Cases of pancreatitis, possibly secondary to biliary obstruction, have been reported rarely in patients treated with ceftriaxone. Most patients presented with risk factors for biliary stasis and biliary sludge (preceding major therapy, severe illness, total parenteral nutrition). A cofactor role of ceftriaxone-related biliary precipitation cannot be ruled out.
Information for Patients
Patients should be counseled that antibacterial drugs including ceftriaxone for injection should only be used to treat bacterial infections. They do not treat viral infections (e.g., common cold). When ceftriaxone for injection is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by ceftriaxone for injection or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Considering the maximum duration of treatment and the class of the compound, carcinogenicity studies with ceftriaxone in animals have not been performed. The maximum duration of animal toxicity studies was 6 months.
Pregnancy
Teratogenic Effects
Pregnancy Category B
Reproductive studies have been performed in mice and rats at doses up to 20 times the usual human dose and have no evidence of embryotoxicity, fetotoxicity or teratogenicity. In primates, no embryotoxicity or teratogenicity was demonstrated at a dose approximately 3 times the human dose.
There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproductive studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nonteratogenic Effects
In rats, in the Segment I (fertility and general reproduction) and Segment III (perinatal and postnatal) studies with intravenously administered ceftriaxone, no adverse effects were noted on various reproductive parameters during gestation and lactation, including postnatal growth, functional behavior and reproductive ability of the offspring, at doses of 586 mg/kg/day or less.
Nursing Mothers
Low concentrations of ceftriaxone are excreted in human milk. Caution should be exercised when ceftriaxone is administered to a nursing woman.
Pediatric Use
Safety and effectiveness of ceftriaxone in neonates, infants and pediatric patients have been established for the dosages described in the DOSAGE AND ADMINISTRATION section. In vitro studies have shown that ceftriaxone, like some other cephalosporins, can displace bilirubin from serum albumin. Ceftriaxone should not be administered to hyperbilirubinemic neonates, especially prematures (see CONTRAINDICATIONS).
Geriatric Use
Of the total number of subjects in clinical studies of ceftriaxone, 32% were 60 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
The pharmacokinetics of ceftriaxone were only minimally altered in geriatric patients compared to healthy adult subjects and dosage adjustments are not necessary for geriatric patients with ceftriaxone dosages up to 2 grams per day (see CLINICAL PHARMACOLOGY).
-
ADVERSE REACTIONS
Ceftriaxone is generally well tolerated. In clinical trials, the following adverse reactions, which were considered to be related to ceftriaxone therapy or of uncertain etiology, were observed:
Local Reactions - pain, induration and tenderness was 1% overall. Phlebitis was reported in <1% after IV administration. The incidence of warmth, tightness or induration was 17% (3/17) after IM administration of 350 mg/mL and 5% (1/20) after IM administration of 250 mg/mL.
Hypersensitivity - rash (1.7%). Less frequently reported (<1 %) were pruritus, fever or chills.
Hematologic - eosinophilia (6%), thrombocytosis (5.1%) and leukopenia (2.1%). Less frequently reported (<1%) were anemia, hemolytic anemia, neutropenia, lymphopenia, thrombocytopenia and prolongation of the prothrombin time.
Gastrointestinal - diarrhea (2.7%). Less frequently reported (<1%) were nausea or vomiting, and dysgeusia. The onset of pseudomembranous colitis symptoms may occur during or after antibacterial treatment (see WARNINGS).
Hepatic - elevations of SGOT (3.1%) or SGPT (3.3%). Less frequently reported (<1 %) were elevations of alkaline phosphatase and bilirubin.
Renal - elevations of the BUN (1.2%). Less frequently reported (<1 %) were elevations of creatinine and the presence of casts in the urine.
Central Nervous System - headache or dizziness were reported occasionally (<1 %).
Genitourinary - moniliasis or vaginitis were reported occasionally (<1%).
Miscellaneous - diaphoresis and flushing were reported occasionally (< 1%).
Other rarely observed adverse reactions (< 0.1%) include abdominal pain, agranulocytosis, allergic pneumonitis, anaphylaxis, basophilia, biliary lithiasis, bronchospasm, colitis, dyspepsia, epistaxis, flatulence, gallbladder sludge, glycosuria, hematuria, jaundice, leukocytosis, lymphocytosis, monocytosis, nephrolithiasis, palpitations, a decrease in the prothrombin time, renal precipitations, seizures, and serum sickness.
Postmarketing Experience: In addition to the adverse reactions reported during clinical trials, the following adverse experiences have been reported during clinical practice in patients treated with ceftriaxone. Data are generally insufficient to allow an estimate of incidence or to establish causation.
A small number of cases of fatal outcomes in which a crystalline material was observed in the lungs and kidneys at autopsy have been reported in neonates receiving ceftriaxone and calcium-containing fluids. In some of these cases, the same intravenous infusion line was used for both ceftriaxone and calcium-containing fluids and in some a precipitate was observed in the intravenous infusion line. At least one fatality has been reported in a neonate in whom ceftriaxone and calcium-containing fluids were administered at different time points via different intravenous lines; no crystalline material was observed at autopsy in this neonate. There have been no similar reports in patients other than neonates.
Gastrointestinal - stomatitis and glossitis.
Genitourinary - oliguria.
Dermatologic– exanthema, allergic dermatitis, urticaria, edema. As with many medications, isolated cases of severe cutaneous adverse reactions (erythema multiforme, Stevens-Johnson syndrome or Lyell’s syndrome/toxic epidermal necrolysis) have been reported.
Cephalosporin Class Adverse Reactions
In addition to the adverse reactions listed above which have been observed in patients treated with ceftriaxone, the following adverse reactions and altered laboratory test results have been reported for cephalosporin class antibiotics:
Adverse Reactions: Allergic reactions, drug fever, serum sickness-like reaction, renal dysfunction, toxic nephropathy, reversible hyperactivity, hypertonia, hepatic dysfunction including cholestasis, aplastic anemia, hemorrhage, and superinfection.
Altered Laboratory Tests: Positive direct Coombs’ test, false-positive test for urinary glucose, and elevated LDH.
Several cephalosporins have been implicated in triggering seizures, particularly in patients with renal impairment when the dosage was not reduced (see DOSAGE AND ADMINISTRATION). If seizures associated with drug therapy occur, the drug should be discontinued. Anticonvulsant therapy can be given if clinically indicated.
- OVERDOSAGE
-
DOSAGE & ADMINISTRATION
Ceftriaxone sodium may be administered intravenously or intramuscularly.
Do not use diluents containing calcium, such as Ringer’s solution or Hartmann’s solution, to reconstitute ceftriaxone vials or to further dilute a reconstituted vial for IV administration because a precipitate can form. Precipitation of ceftriaxonecalcium can also occur when ceftriaxone is mixed with calcium-containing solutions in the same IV administration line. Ceftriaxone must not be administered simultaneously with calcium-containing IV solutions, including continuous calcium-containing infusions such as parenteral nutrition via a Y-site. However, in patients other than neonates, ceftriaxone and calcium-containing solutions may be administered sequentially of one another if the infusion lines are thoroughly flushed between infusions with a compatible fluid (see WARNINGS).
There have been no reports of an interaction between ceftriaxone and oral calcium-containing products or interaction between intramuscular ceftriaxone and calcium-containing products (IV or oral).
Neonates
Hyperbilirubinemic neonates, especially prematures, should not be treated with ceftriaxone (see CONTRAINDICATIONS).
Ceftriaxone is contraindicated in neonates if they require (or are expected to require) treatment with calcium-containing IV solutions, including continuous calcium-containing infusions such as parenteral nutrition because of the risk of precipitation of ceftriaxone- calcium (see CONTRAINDICATIONS).
Pediatric Patients
For the treatment of skin and skin structure infections, the recommended total daily dose is 50 to 75 mg/kg given once a day (or in equally divided doses twice a day). The total daily dose should not exceed 2 grams.
For the treatment of acute bacterial otitis media, a single intramuscular dose of 50 mg/kg (not to exceed 1 gram) is recommended (see INDICATIONS AND USAGE).
For the treatment of serious miscellaneous infections other than meningitis, the recommended total daily dose is 50 to 75 mg/kg, given in divided doses every 12 hours. The total daily dose should not exceed 2 grams.
In the treatment of meningitis, it is recommended that the initial therapeutic dose be 100 mg/kg (not to exceed 4 grams). Thereafter, a total daily dose of 100 mg/kg/day (not to exceed 4 grams daily) is recommended. The daily dose may be administered once a day (or in equally divided doses every 12 hours). The usual duration of therapy is 7 to 14 days.
Adults
The usual adult daily dose is 1 to 2 grams given once a day (or in equally divided doses twice a day) depending on the type and severity of infection. The total daily dose should not exceed 4 grams.
If Chlamydia trachomatis is a suspected pathogen, appropriate antichlamydial coverage should be added, because ceftriaxone sodium has no activity against this organism.
For the treatment of uncomplicated gonococcal infections, a single intramuscular dose of 250 mg is recommended.
For preoperative use (surgical prophylaxis), a single dose of 1 gram administered intravenously 1/2 to 2 hours before surgery is recommended.
Generally, ceftriaxone therapy should be continued for at least 2 days after the signs and symptoms of infection have disappeared. The usual duration of therapy is 4 to 14 days; in complicated infections, longer therapy may be required.
When treating infections caused by Streptococcus pyogenes, therapy should be continued for at least 10 days.
No dosage adjustment is necessary for patients with impairment of renal or hepatic function.
DIRECTIONS FOR USE
Intramuscular Administration
Reconstitute ceftriaxone sodium powder with the appropriate diluent (see COMPATIBILITY AND STABILITY).
Inject diluent into vial, shake vial thoroughly to form solution. Withdraw entire contents of vial into syringe to equal total labeled dose.
After reconstitution, each 1 mL of solution contains approximately 250 mg or 350 mg equivalent of ceftriaxone according to the amount of diluent indicated below. If required, more dilute solutions could be utilized. A 350 mg/mL concentration is not recommended for the 250 mg vial since it may not be possible to withdraw the entire contents.
As with all intramuscular preparations, ceftriaxone sodium should be injected well within the body of a relatively large muscle; aspiration helps to avoid unintentional injection into a blood vessel.
Vial Dosage Size Amount of Diluent to be Added 250 mg/mL 350 mg/mL 250 mg 0.9 mL - 500 mg 1.8 mL 1 mL 1 gm 3.6 mL 2.1 mL 2 gm 7.2 mL 4.2 mL Intravenous Administration
Ceftriaxone sodium should be administered intravenously by infusion over a period of 30 minutes. Concentrations between 10 mg/mL and 40 mg/mL are recommended; however, lower concentrations may be used if desired. Reconstitute vials or “piggyback” bottles with an appropriate IV diluent (see COMPATIBILITY AND STABILITY).
Vial Dosage Size Amount of Diluent to be Added 250 mg 2.4 mL 500 mg 4.8 mL 1 gm 9.6 mL 2 gm 19.2 mL After reconstitution, each 1 mL of solution contains approximately 100 mg equivalent of ceftriaxone. Withdraw entire contents and dilute to the desired concentration with the appropriate IV diluent.
Piggyback Bottle Dosage Size Amount of Diluent to be Added 1 gm 10 mL 2 gm 20 mL After reconstitution, further dilute to 50 mL or 100 mL volumes with the appropriate IV diluent.
COMPATIBILITY AND STABILITY
Ceftriaxone has been shown to be compatible with Flagyl ®18 IV (metronidazole hydrochloride). The concentration should not exceed 5 to 7.5 mg/mL metronidazole hydrochloride with ceftriaxone 10 mg/mL as an admixture. The admixture is stable for 24 hours at room temperature only in 0.9% sodium chloride injection or 5% dextrose in water (D5W). No compatibility studies have been conducted with the Flagyl® IV RTU® (metronidazole) formulation or using other diluents. Metronidazole at concentrations greater than 8 mg/mL will precipitate. Do not refrigerate the admixture as precipitation will occur.
Vancomycin, amsacrine, aminoglycosides, and fluconazole are physically incompatible with ceftriaxone in admixtures. When any of these drugs are to be administered concomitantly with ceftriaxone by intermittent intravenous infusion, it is recommended that they be given sequentially, with thorough flushing of the intravenous lines (with one of the compatible fluids) between the administrations.
Do not use diluents containing calcium, such as Ringer’s solution or Hartmann’s solution, to reconstitute ceftriaxone vials or to further dilute a reconstituted vial for IV administration. Particulate formation can result.
Ceftriaxone sodium solutions should not be physically mixed with or piggybacked into solutions containing other antimicrobial drugs or into diluent solutions other than those listed above, due to possible incompatibility (see WARNINGS).
Ceftriaxone sodium sterile powder should be stored at 20°-25°C (68°-77°F) [See USP Controlled Room Temperature] and protected from light. After reconstitution, protection from normal light is not necessary. The color of solutions ranges from light yellow to amber, depending on the length of storage, concentration and diluent used.
Ceftriaxone sodium intramuscular solutions remain stable (loss of potency less than 10%) for the following time periods:
Storage Diluent
Concentration
mg/mLRoom Temp.
(25°C)Refrigerated
(4°C)Sterile Water for Injection 100 2 days 10 days 250, 350 24 hours 3 days 0.9% Sodium Chloride
Solution100 2 days 10 days 250, 350 24 hours 3 days 5% Dextrose Solution 100 2 days 10 days 250, 350 24 hours 3 days Bacteriostatic Water + 0.9%
Benzyl Alcohol100 24 hours 10 days 250, 350 24 hours 3 days 1% Lidocaine Solution
(without epinephrine)100 24 hours 10 days 250, 350 24 hours 3 days Ceftriaxone sodium intravenous solutions, at concentrations of 10, 20 and 40 mg/mL, remain stable (loss of potency less than 10%) for the following time periods stored in glass or PVC containers:
Storage - * Data available for 10 to 40 mg/mL concentrations in this diluent in PVC containers only.
Diluent
Room Temp.
(25°C)Refrigerated
(4°C)Sterile Water 2 days 10 days 0.9% Sodium Chloride Solution 2 days 10 days 5% Dextrose Solution 2 days 10 days 10% Dextrose Solution 2 days 10 days 5% Dextrose + 0.9% Sodium Chloride Solution* 2 days Incompatible 5% Dextrose + 0.45% Sodium Chloride Solution 2 days Incompatible Similarly, ceftriaxone sodium intravenous solutions, at concentrations of 100 mg/mL, remain stable in the IV piggyback glass containers for the above specified time periods.
The following intravenous ceftriaxone sodium solutions are stable at room temperature (25°C) for 24 hours, at concentrations between 10 mg/mL and 40 mg/mL: Sodium Lactate (PVC container), 10% Invert Sugar (glass container), 5% Sodium Bicarbonate (glass container), Freamine III (glass container), Normosol-M in 5% Dextrose (glass and PVC containers), Ionosol-B in 5% Dextrose (glass container), 5% Mannitol (glass container), 10% Mannitol (glass container).
After the indicated stability time periods, unused portions of solutions should be discarded.
NOTE: Parenteral drug products should be inspected visually for particulate matter before administration.
Ceftriaxone sodium reconstituted with 5% Dextrose or 0.9% Sodium Chloride solution at concentrations between 10 mg/mL and 40 mg/mL, and then stored in frozen state (-20°C) in PVC or polyolefin containers, remains stable for 26 weeks.
Frozen solutions of ceftriaxone sodium should be thawed at room temperature before use. After thawing, unused portions should be discarded. DO NOT REFREEZE.
- 18
Registered trademark of G.D. Searle & Co.
-
ANIMAL PHARMACOLOGY
Concretions consisting of the precipitated calcium salt of ceftriaxone have been found in the gallbladder bile of dogs and baboons treated with ceftriaxone.
These appeared as a gritty sediment in dogs that received 100 mg/kg/day for 4 weeks. A similar phenomenon has been observed in baboons but only after a protracted dosing period (6 months) at higher dose levels (335 mg/kg/day or more). The likelihood of this occurrence in humans is considered to be low, since ceftriaxone has a greater plasma half-life in humans, the calcium salt of ceftriaxone is more soluble in human gallbladder bile and the calcium content of human gallbladder bile is relatively low.
-
HOW SUPPLIED
Ceftriaxone sodium is supplied as a sterile crystalline powder in glass vials and piggyback bottles. The following packages are available:
Vials containing 500 mg equivalent of ceftriaxone. Box of 10 (NDC: 21695-202-10) .
Vials containing 1 gm equivalent of ceftriaxone. Box of 1 (NDC: 21695-352-01).
Vials containing 1 gm equivalent of ceftriaxone. Box of 10 (NDC: 21695-352-10).
NOTE: Ceftriaxone sodium sterile powder should be stored at 20° - 25°C (68° - 77°F) [See USP Controlled Room Temperature] and protected from light.
-
CLINICAL STUDIES
Clinical Trials in Pediatric Patients With Acute Bacterial Otitis Media
In two adequate and well-controlled U.S. clinical trials a single IM dose of ceftriaxone was compared with a 10 day course of oral antibiotic in pediatric patients between the ages of 3 months and 6 years. The clinical cure rates and statistical outcome appear in the table below:
Clinical Efficacy in Evaluable Population - * Barnett ED, Teele DW, Klein JO, et al. Comparison of Ceftriaxone and Trimethoprim- Sulfamethoxazole for Acute Otitis Media. Pediatrics. Vol .99, No. 1, January 1997.
Study Day Ceftriaxone Single Dose Comparator- 10 Days of Oral Therapy 95 % Confidence Interval Statistical Outcome Study 1 - U.S. amoxicillin/clavulanate 14 74% (220/296) 82% (247/302) (-14.4%, -0.5%) Ceftriaxone is lower than control at study day 14 and 28. 28 58% (167/288) 67% (200/297) (-17.5%, -1.2%) Study 2 - U.S.* TMP-SMZ 14 54% (113/210) 60% (124/206) (-16.4%, 3.6%) Ceftriaxone is equivalent to control at study day 14 and 28. 28 35% (73/206) 45% (93/205) (-19.9%, 0.0%) An open-label bacteriologic study of ceftriaxone without a comparator enrolled 108 pediatric patients, 79 of whom had positive baseline cultures for one or more of the common pathogens. The results of this study are tabulated as follows:
Week 2 and 4 Bacteriologic Eradication Rates in the Per Protocol Analysis in the Roche Bacteriologic Study by pathogen:
Study Day
13-15Study Day
30+2Organism No. Analyzed No. Erad. (%) No. Analyzed No. Erad. (%) Streptococcus pneumoniae 38 32 (84) 35 25 (71) Haemophilus influenzae 33 28 (85) 31 22 (71) Moraxella catarrhalis 15 12 (80) 15 9 (60) -
REFERENCES
- National Committee for Clinical Laboratory Standards, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard-Fifth Edition. NCCLS document M7-A5 (ISBN 1-56238-309-9).NCCLS, Wayne, PA 19087-1898, 2000.
- National Committee for Clinical Laboratory Standards, Supplemental Tables. NCCLS document M100-S10 (M7) (ISBN 1-56238-309-9). NCCLS, Wayne, PA 19087-1898, 2000.
- National Committee for Clinical Laboratory Standards, Performance Standards for Antimicrobial Disc Susceptibility Tests; Approved Standard - Seventh Edition. NCCLS document M2-A7 (ISBN 1-56238-393-0). NCCLS, Wayne, PA 19087-1898, 2000.
- National Committee for Clinical Laboratory Standards, Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard- Fourth Edition. NCCLS document M11-A4 (ISBN 1- 56238-210-1). NCCLS, Wayne, PA 19087-1898, 1997.
- Barnett ED, Teele DW, Klein JO, et al. Comparison of Ceftriaxone and Trimethoprim- Sulfamethoxazole for Acute Otitis Media. Pediatrics. Vol. 99, No. 1, January 1997.
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
CEFTRIAXONE SODIUM
ceftriaxone sodium injection, powder, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 21695-202(NDC: 60505-751) Route of Administration INTRAVENOUS, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CEFTRIAXONE SODIUM (UNII: 023Z5BR09K) (CEFTRIAXONE - UNII:75J73V1629) CEFTRIAXONE 500 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 21695-202-10 10 in 1 BOX 1 1 in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065230 08/02/2005 CEFTRIAXONE SODIUM
ceftriaxone sodium injection, powder, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 21695-352(NDC: 60505-752) Route of Administration INTRAVENOUS, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CEFTRIAXONE SODIUM (UNII: 023Z5BR09K) (CEFTRIAXONE - UNII:75J73V1629) CEFTRIAXONE 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 21695-352-10 10 in 1 BOX 1 1 in 1 VIAL 2 NDC: 21695-352-01 1 in 1 BOX Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065230 08/02/2005 Labeler - Rebel Distributors Corp (118802834) Establishment Name Address ID/FEI Business Operations Rebel Distributors Corp 118802834 RELABEL, REPACK
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.