These highlights do not include all the information needed to use VALTREX safely and effectively. See full prescribing information for VALTREX. VALTREX (valacyclovir hydrochloride) CapletsInitial U.S. Approval: 1995
VALTREX by
Drug Labeling and Warnings
VALTREX by is a Prescription medication manufactured, distributed, or labeled by A-S Medication Solutions. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
VALTREX- valacyclovir hydrochloride tablet, film coated
A-S Medication Solutions
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VALTREX safely and effectively. See full prescribing information for VALTREX.
VALTREX (valacyclovir hydrochloride) Caplets Initial U.S. Approval: 1995 INDICATIONS AND USAGEVALTREX is a nucleoside analogue DNA polymerase inhibitor indicated for: Adult Patients (1.1)
Pediatric Patients (1.2)
Limitations of Use (1.3)
DOSAGE AND ADMINISTRATION
Valacyclovir oral suspension (25 mg/mL or 50 mg/mL) can be prepared from the 500 mg VALTREX Caplets. (2.3) DOSAGE FORMS AND STRENGTHSCaplets: 500 mg (unscored), 1 gram (partially scored) (3) CONTRAINDICATIONSHypersensitivity to valacyclovir (e.g., anaphylaxis), acyclovir, or any component of the formulation. (4) WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 1/2020 |
|||||||||||||||||||||||||||||
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Adult Patients
Cold Sores (Herpes Labialis): VALTREX® (valacyclovir hydrochloride) Caplets are indicated for treatment of cold sores (herpes labialis). The efficacy of VALTREX initiated after the development of clinical signs of a cold sore (e.g., papule, vesicle, or ulcer) has not been established.
Genital Herpes: Initial Episode: VALTREX is indicated for treatment of the initial episode of genital herpes in immunocompetent adults. The efficacy of treatment with VALTREX when initiated more than 72 hours after the onset of signs and symptoms has not been established.
Recurrent Episodes: VALTREX is indicated for treatment of recurrent episodes of genital herpes in immunocompetent adults. The efficacy of treatment with VALTREX when initiated more than 24 hours after the onset of signs and symptoms has not been established.
Suppressive Therapy: VALTREX is indicated for chronic suppressive therapy of recurrent episodes of genital herpes in immunocompetent and in HIV-1─infected adults. The efficacy and safety of VALTREX for the suppression of genital herpes beyond 1 year in immunocompetent patients and beyond 6 months in HIV-1─infected patients have not been established.
Reduction of Transmission: VALTREX is indicated for the reduction of transmission of genital herpes in immunocompetent adults. The efficacy of VALTREX for the reduction of transmission of genital herpes beyond 8 months in discordant couples has not been established. The efficacy of VALTREX for the reduction of transmission of genital herpes in individuals with multiple partners and non─heterosexual couples has not been established. Safer sex practices should be used with suppressive therapy (see current Centers for Disease Control and Prevention [CDC] Sexually Transmitted Diseases Treatment Guidelines).
Herpes Zoster: VALTREX is indicated for the treatment of herpes zoster (shingles) in immunocompetent adults. The efficacy of VALTREX when initiated more than 72 hours after the onset of rash and the efficacy and safety of VALTREX for treatment of disseminated herpes zoster have not been established.
1.2 Pediatric Patients
Cold Sores (Herpes Labialis): VALTREX is indicated for the treatment of cold sores (herpes labialis) in pediatric patients aged greater than or equal to 12 years. The efficacy of VALTREX initiated after the development of clinical signs of a cold sore (e.g., papule, vesicle, or ulcer) has not been established.
Chickenpox: VALTREX is indicated for the treatment of chickenpox in immunocompetent pediatric patients aged 2 to less than 18 years. Based on efficacy data from clinical trials with oral acyclovir, treatment with VALTREX should be initiated within 24 hours after the onset of rash [see Clinical Studies (14.4)].
1.3 Limitations of Use
The efficacy and safety of VALTREX have not been established in:
- Immunocompromised patients other than for the suppression of genital herpes in HIV─1─infected patients with a CD4+ cell count greater than or equal to 100 cells/mm3.
- Patients aged less than 12 years with cold sores (herpes labialis).
- Patients aged less than 2 years or greater than or equal to 18 years with chickenpox.
- Patients aged less than 18 years with genital herpes.
- Patients aged less than 18 years with herpes zoster.
- Neonates and infants as suppressive therapy following neonatal herpes simplex virus (HSV) infection.
2 DOSAGE AND ADMINISTRATION
- VALTREX may be given without regard to meals.
- Valacyclovir oral suspension (25 mg/mL or 50 mg/mL) may be prepared extemporaneously from 500-mg VALTREX Caplets for use in pediatric patients for whom a solid dosage form is not appropriate [see Dosage and Administration (2.3)].
2.1 Adult Dosing Recommendations
Cold Sores (Herpes Labialis): The recommended dosage of VALTREX for treatment of cold sores is 2 grams twice daily for 1 day taken 12 hours apart. Therapy should be initiated at the earliest symptom of a cold sore (e.g., tingling, itching, or burning).
Genital Herpes: Initial Episode: The recommended dosage of VALTREX for treatment of initial genital herpes is 1 gram twice daily for 10 days. Therapy was most effective when administered within 48 hours of the onset of signs and symptoms.
Recurrent Episodes: The recommended dosage of VALTREX for treatment of recurrent genital herpes is 500 mg twice daily for 3 days. Initiate treatment at the first sign or symptom of an episode.
Suppressive Therapy: The recommended dosage of VALTREX for chronic suppressive therapy of recurrent genital herpes is 1 gram once daily in patients with normal immune function. In patients with a history of 9 or fewer recurrences per year, an alternative dose is 500 mg once daily.
In HIV─1─infected patients with a CD4+ cell count greater than or equal to 100 cells/mm3, the recommended dosage of VALTREX for chronic suppressive therapy of recurrent genital herpes is 500 mg twice daily.
Reduction of Transmission: The recommended dosage of VALTREX for reduction of transmission of genital herpes in patients with a history of 9 or fewer recurrences per year is 500 mg once daily for the source partner.
Herpes Zoster: The recommended dosage of VALTREX for treatment of herpes zoster is 1 gram 3 times daily for 7 days. Therapy should be initiated at the earliest sign or symptom of herpes zoster and is most effective when started within 48 hours of the onset of rash.
2.2 Pediatric Dosing Recommendations
Cold Sores (Herpes Labialis): The recommended dosage of VALTREX for the treatment of cold sores in pediatric patients aged greater than or equal to 12 years is 2 grams twice daily for 1 day taken 12 hours apart. Therapy should be initiated at the earliest symptom of a cold sore (e.g., tingling, itching, or burning).
Chickenpox: The recommended dosage of VALTREX for treatment of chickenpox in immunocompetent pediatric patients aged 2 to less than 18 years is 20 mg/kg administered 3 times daily for 5 days. The total dose should not exceed 1 gram 3 times daily. Therapy should be initiated at the earliest sign or symptom [see Use in Specific Populations (8.4), Clinical Pharmacology (12.3), Clinical Studies (14.4)].
2.3 Extemporaneous Preparation of Oral Suspension
Ingredients and Preparation per USP─NF: VALTREX Caplets 500 mg, cherry flavor, and Suspension Structured Vehicle USP─NF (SSV). Valacyclovir oral suspension (25 mg/mL or 50 mg/mL) should be prepared in lots of 100 mL.
Prepare Suspension at Time of Dispensing as Follows:
- Prepare SSV according to the USP-NF.
- Using a pestle and mortar, grind the required number of VALTREX 500 mg Caplets until a fine powder is produced (5 VALTREX Caplets for 25 mg/mL suspension; 10 VALTREX Caplets for 50 mg/mL suspension).
- Gradually add approximately 5-mL aliquots of SSV to the mortar and triturate the powder until a paste has been produced. Ensure that the powder has been adequately wetted.
- Continue to add approximately 5-mL aliquots of SSV to the mortar, mixing thoroughly between additions, until a concentrated suspension is produced, to a minimum total quantity of 20 mL SSV and a maximum total quantity of 40 mL SSV for both the 25-mg/mL and 50─mg/mL suspensions.
- Transfer the mixture to a suitable 100-mL measuring flask.
- Transfer the cherry flavor* to the mortar and dissolve in approximately 5 mL of SSV. Once dissolved, add to the measuring flask.
- Rinse the mortar at least 3 times with approximately 5-mL aliquots of SSV, transferring the rinsing to the measuring flask between additions.
- Make the suspension to volume (100 mL) with SSV and shake thoroughly to mix.
- Transfer the suspension to an amber glass medicine bottle with a child─resistant closure.
- The prepared suspension should be labeled with the following information “Shake well before using. Store suspension between 2° to 8°C (36° to 46°F) in a refrigerator. Discard after 28 days.”
*The amount of cherry flavor added is as instructed by the suppliers of the cherry flavor.
2.4 Patients With Renal Impairment
Dosage recommendations for adult patients with reduced renal function are provided in Table 1 [see Use in Specific Populations (8.5, 8.6), Clinical Pharmacology (12.3)]. Data are not available for the use of VALTREX in pediatric patients with a creatinine clearance less than 50 mL/min/1.73 m2.
|
Indications |
Normal Dosage Regimen (Creatinine Clearance ≥50 mL/min) |
Creatinine Clearance (mL/min) |
||
|
30-49 |
10-29 |
<10 |
||
|
Cold sores (Herpes labialis)
Do not exceed 1 day of treatment. |
Two 2 gram doses taken 12 hours apart |
Two 1 gram doses taken 12 hours apart |
Two 500 mg doses taken 12 hours apart |
500 mg single dose |
|
Genital herpes: Initial episode |
1 gram every 12 hours |
no reduction |
1 gram every 24 hours |
500 mg every 24 hours |
|
Genital herpes: Recurrent episode |
500 mg every 12 hours |
no reduction |
500 mg every 24 hours |
500 mg every 24 hours |
|
Genital herpes: Suppressive therapy | ||||
|
Immunocompetent patients
|
1 gram every 24 hours |
no reduction |
500 mg every 24 hours |
500 mg every 24 hours |
|
Alternate dose for immunocompetent patients with less than or equal to 9 recurrences/year |
500 mg every 24 hours |
no reduction |
500 mg every 48 hours |
500 mg every 48 hours |
|
HIV─1─infected patients |
500 mg every 12 hours |
no reduction |
500 mg every 24 hours |
500 mg every 24 hours |
|
Herpes zoster |
1 gram every 8 hours |
1 gram every 12 hours |
1 gram every 24 hours |
500 mg every 24 hours |
Hemodialysis: Patients requiring hemodialysis should receive the recommended dose of VALTREX after hemodialysis. During hemodialysis, the half─life of acyclovir after administration of VALTREX is approximately 4 hours. About one-third of acyclovir in the body is removed by dialysis during a 4─hour hemodialysis session.
Peritoneal Dialysis: There is no information specific to administration of VALTREX in patients receiving peritoneal dialysis. The effect of chronic ambulatory peritoneal dialysis (CAPD) and continuous arteriovenous hemofiltration/dialysis (CAVHD) on acyclovir pharmacokinetics has been studied. The removal of acyclovir after CAPD and CAVHD is less pronounced than with hemodialysis, and the pharmacokinetic parameters closely resemble those observed in patients with end─stage renal disease (ESRD) not receiving hemodialysis. Therefore, supplemental doses of VALTREX should not be required following CAPD or CAVHD.
3 DOSAGE FORMS AND STRENGTHS
Caplets:
- 500-mg: blue, film─coated, capsule─shaped tablets printed with "VALTREX 500 mg."
- 1-gram: blue, film─coated, capsule─shaped tablets, with a partial scorebar on both sides, printed with "VALTREX 1 gram."
4 CONTRAINDICATIONS
VALTREX is contraindicated in patients who have had a demonstrated clinically significant hypersensitivity reaction (e.g., anaphylaxis) to valacyclovir, acyclovir, or any component of the formulation [see Adverse Reactions (6.3)].
5 WARNINGS AND PRECAUTIONS
5.1 Thrombotic Thrombocytopenic Purpura/Hemolytic Uremic Syndrome (TTP/HUS)
TTP/HUS, in some cases resulting in death, has occurred in patients with advanced HIV─1 disease and also in allogeneic bone marrow transplant and renal transplant recipients participating in clinical trials of VALTREX at doses of 8 grams per day. Treatment with VALTREX should be stopped immediately if clinical signs, symptoms, and laboratory abnormalities consistent with TTP/HUS occur.
5.2 Acute Renal Failure
Cases of acute renal failure have been reported in:
- Elderly patients with or without reduced renal function. Caution should be exercised when administering VALTREX to geriatric patients, and dosage reduction is recommended for those with impaired renal function [see Dosage and Administration (2.4), Use in Specific Populations (8.5)].
- Patients with underlying renal disease who received higher-than-recommended doses of VALTREX for their level of renal function. Dosage reduction is recommended when administering VALTREX to patients with renal impairment [see Dosage and Administration (2.4), Use in Specific Populations (8.6)].
- Patients receiving other nephrotoxic drugs. Caution should be exercised when administering VALTREX to patients receiving potentially nephrotoxic drugs.
- Patients without adequate hydration. Precipitation of acyclovir in renal tubules may occur when the solubility (2.5 mg/mL) is exceeded in the intratubular fluid. Adequate hydration should be maintained for all patients.
In the event of acute renal failure and anuria, the patient may benefit from hemodialysis until renal function is restored [see Dosage and Administration (2.4), Adverse Reactions (6.3)].
5.3 Central Nervous System Effects
Central nervous system adverse reactions, including agitation, hallucinations, confusion, delirium, seizures, and encephalopathy, have been reported in both adult and pediatric patients with or without reduced renal function and in patients with underlying renal disease who received higher-than-recommended doses of VALTREX for their level of renal function. Elderly patients are more likely to have central nervous system adverse reactions. VALTREX should be discontinued if central nervous system adverse reactions occur [see Adverse Reactions (6.3), Use in Specific Populations (8.5, 8.6)].
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of the labeling:
- Thrombotic Thrombocytopenic Purpura/Hemolytic Uremic Syndrome [see Warnings and Precautions (5.1)].
- Acute Renal Failure [see Warnings and Precautions (5.2)].
- Central Nervous System Effects [see Warnings and Precautions (5.3)].
The most common adverse reactions reported in at least 1 indication by greater than 10% of adult subjects treated with VALTREX and observed more frequently with VALTREX compared to placebo are headache, nausea, and abdominal pain. The only adverse reaction reported in greater than 10% of pediatric subjects aged less than 18 years was headache.
6.1 Clinical Trials Experience in Adult Subjects
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Cold Sores (Herpes Labialis): In clinical trials for the treatment of cold sores, the adverse reactions reported by subjects receiving VALTREX 2 grams twice daily (n = 609) or placebo (n = 609) for 1 day, respectively, included headache (14%, 10%) and dizziness (2%, 1%). The frequencies of abnormal ALT (greater than 2 x ULN) were 1.8% for subjects receiving VALTREX compared with 0.8% for placebo. Other laboratory abnormalities (hemoglobin, white blood cells, alkaline phosphatase, and serum creatinine) occurred with similar frequencies in the 2 groups.
Genital Herpes: Initial Episode: In a clinical trial for the treatment of initial episodes of genital herpes, the adverse reactions reported by greater than or equal to 5% of subjects receiving VALTREX 1 gram twice daily for 10 days (n = 318) or oral acyclovir 200 mg 5 times daily for 10 days (n = 318), respectively, included headache (13%, 10%) and nausea (6%, 6%). For the incidence of laboratory abnormalities see Table 2.
Recurrent Episodes: In 3 clinical trials for the episodic treatment of recurrent genital herpes, the adverse reactions reported by greater than or equal to 5% of subjects receiving VALTREX 500 mg twice daily for 3 days (n = 402), VALTREX 500 mg twice daily for 5 days (n = 1,136) or placebo (n = 259), respectively, included headache (16%, 11%, 14%) and nausea (5%, 4%, 5%). For the incidence of laboratory abnormalities see Table 2.
Suppressive Therapy: Suppression of Recurrent Genital Herpes in Immunocompetent Adults: In a clinical trial for the suppression of recurrent genital herpes infections, the adverse reactions reported by subjects receiving VALTREX 1 gram once daily (n = 269), VALTREX 500 mg once daily (n = 266), or placebo (n = 134), respectively, included headache (35%, 38%, 34%), nausea (11%, 11%, 8%), abdominal pain (11%, 9%, 6%), dysmenorrhea (8%, 5%, 4%), depression (7%, 5%, 5%), arthralgia (6%, 5%, 4%), vomiting (3%, 3%, 2%), and dizziness (4%, 2%, 1%). For the incidence of laboratory abnormalities see Table 2.
Suppression of Recurrent Genital Herpes in HIV─1-Infected Subjects: In HIV─1-infected subjects, frequently reported adverse reactions for VALTREX (500 mg twice daily; n = 194, median days on therapy = 172) and placebo (n = 99, median days on therapy = 59), respectively, included headache (13%, 8%), fatigue (8%, 5%), and rash (8%, 1%). Post─randomization laboratory abnormalities that were reported more frequently in valacyclovir subjects versus placebo included elevated alkaline phosphatase (4%, 2%), elevated ALT (14%, 10%), elevated AST (16%, 11%), decreased neutrophil counts (18%, 10%), and decreased platelet counts (3%, 0%), respectively.
Reduction of Transmission: In a clinical trial for the reduction of transmission of genital herpes, the adverse reactions reported by subjects receiving VALTREX 500 mg once daily (n = 743) or placebo once daily (n = 741), respectively, included headache (29%, 26%), nasopharyngitis (16%, 15%), and upper respiratory tract infection (9%, 10%).
Herpes Zoster: In 2 clinical trials for the treatment of herpes zoster, the adverse reactions reported by subjects receiving VALTREX 1 gram 3 times daily for 7 to 14 days (n = 967) or placebo (n = 195), respectively, included nausea (15%, 8%), headache (14%, 12%), vomiting (6%, 3%), dizziness (3%, 2%), and abdominal pain (3%, 2%). For the incidence of laboratory abnormalities see Table 2.
|
Laboratory Abnormality |
Herpes Zoster |
Genital Herpes Treatment |
Genital Herpes Suppression |
|||||
|
VALTREX 1 gram 3 Times Daily (n = 967) |
Placebo (n = 195) |
VALTREX 1 gram Twice Daily (n = 1,194) |
VALTREX 500 mg Twice Daily (n = 1,159) |
Placebo (n = 439) |
VALTREX 1 gram Once Daily (n = 269) |
VALTREX 500 mg Once Daily (n = 266) |
Placebo (n = 134) |
|
|
Hemoglobin (<0.8 x LLN) |
0.8% |
0% |
0.3% |
0.2% |
0% |
0% |
0.8% |
0.8% |
|
White blood cells (<0.75 x LLN) |
1.3% |
0.6% |
0.7% |
0.6% |
0.2% |
0.7% |
0.8% |
1.5% |
|
Platelet count (<100,000/mm3) |
1.0% |
1.2% |
0.3% |
0.1% |
0.7% |
0.4% |
1.1% |
1.5% |
|
AST (SGOT) (>2 x ULN) |
1.0% |
0% |
1.0% |
a |
0.5% |
4.1% |
3.8% |
3.0% |
|
Serum creatinine (>1.5 x ULN) |
0.2% |
0% |
0.7% |
0% |
0% |
0% |
0% |
0% |
|
a Data were not collected prospectively. |
||||||||
|
LLN = Lower limit of normal. |
||||||||
|
ULN = Upper limit of normal. |
||||||||
6.2 Clinical Trials Experience in Pediatric Subjects
The safety profile of VALTREX has been studied in 177 pediatric subjects aged 1 month to less than 18 years. Sixty-five of these pediatric subjects, aged 12 to less than 18 years, received oral caplets for 1 to 2 days for treatment of cold sores. The remaining 112 pediatric subjects, aged 1 month to less than 12 years, participated in 3 pharmacokinetic and safety trials and received valacyclovir oral suspension. Fifty-one of these 112 pediatric subjects received oral suspension for 3 to 6 days. The frequency, intensity, and nature of clinical adverse reactions and laboratory abnormalities were similar to those seen in adults.
Pediatric Subjects Aged 12 to Less Than 18 Years (Cold Sores): In clinical trials for the treatment of cold sores, the adverse reactions reported by adolescent subjects receiving VALTREX 2 grams twice daily for 1 day, or VALTREX 2 grams twice daily for 1 day followed by 1 gram twice daily for 1 day (n = 65, across both dosing groups), or placebo (n = 30), respectively, included headache (17%, 3%) and nausea (8%, 0%).
Pediatric Subjects Aged 1 Month to Less Than 12 Years: Adverse events reported in more than 1 subject across the 3 pharmacokinetic and safety trials in children aged 1 month to less than 12 years were diarrhea (5%), pyrexia (4%), dehydration (2%), herpes simplex (2%), and rhinorrhea (2%). No clinically meaningful changes in laboratory values were observed.
6.3 Postmarketing Experience
In addition to adverse events reported from clinical trials, the following events have been identified during postmarketing use of VALTREX. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal connection to VALTREX.
General: Facial edema, hypertension, tachycardia.
Allergic: Acute hypersensitivity reactions including anaphylaxis, angioedema, dyspnea, pruritus, rash, and urticaria [see Contraindications (4)].
CNS Symptoms: Aggressive behavior; agitation; ataxia; coma; confusion; decreased consciousness; dysarthria; encephalopathy; mania; and psychosis, including auditory and visual hallucinations, seizures, tremors [see Warnings and Precautions (5.3), Use in Specific Populations (8.5, 8.6)].
Eye: Visual abnormalities.
Gastrointestinal: Diarrhea.
Hepatobiliary Tract and Pancreas: Liver enzyme abnormalities, hepatitis.
Renal: Renal failure, renal pain (may be associated with renal failure) [see Warnings and Precautions (5.2), Use in Specific Populations (8.5, 8.6)].
Hematologic: Thrombocytopenia, aplastic anemia, leukocytoclastic vasculitis, TTP/HUS [see Warnings and Precautions (5.1)].
Skin: Erythema multiforme, rashes including photosensitivity, alopecia.
7 DRUG INTERACTIONS
No clinically significant drug-drug or drug-food interactions with VALTREX are known [see Clinical Pharmacology (12.3)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B. There are no adequate and well─controlled trials of VALTREX or acyclovir in pregnant women. Based on prospective pregnancy registry data on 749 pregnancies, the overall rate of birth defects in infants exposed to acyclovir in-utero appears similar to the rate for infants in the general population. VALTREX should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
A prospective epidemiologic registry of acyclovir use during pregnancy was established in 1984 and completed in April 1999. There were 749 pregnancies followed in women exposed to systemic acyclovir during the first trimester of pregnancy resulting in 756 outcomes. The occurrence rate of birth defects approximates that found in the general population. However, the small size of the registry is insufficient to evaluate the risk for less common defects or to permit reliable or definitive conclusions regarding the safety of acyclovir in pregnant women and their developing fetuses.
Animal reproduction studies performed at oral doses that provided up to 10 and 7 times the human plasma levels during the period of major organogenesis in rats and rabbits, respectively, revealed no evidence of teratogenicity.
8.3 Nursing Mothers
Following oral administration of a 500─mg dose of VALTREX to 5 nursing mothers, peak acyclovir concentrations (Cmax) in breast milk ranged from 0.5 to 2.3 times (median 1.4) the corresponding maternal acyclovir serum concentrations. The acyclovir breast milk AUC ranged from 1.4 to 2.6 times (median 2.2) maternal serum AUC. A 500─mg maternal dosage of VALTREX twice daily would provide a nursing infant with an oral acyclovir dosage of approximately 0.6 mg/kg/day. This would result in less than 2% of the exposure obtained after administration of a standard neonatal dose of 30 mg/kg/day of intravenous acyclovir to the nursing infant. Unchanged valacyclovir was not detected in maternal serum, breast milk, or infant urine. Caution should be exercised when VALTREX is administered to a nursing woman.
8.4 Pediatric Use
VALTREX is indicated for treatment of cold sores in pediatric patients aged greater than or equal to 12 years and for treatment of chickenpox in pediatric patients aged 2 to less than 18 years [see Indications and Usage (1.2), Dosage and Administration (2.2)].
The use of VALTREX for treatment of cold sores is based on 2 double-blind, placebo-controlled clinical trials in healthy adults and adolescents (aged greater than or equal to 12 years) with a history of recurrent cold sores [see Clinical Studies (14.1)].
The use of VALTREX for treatment of chickenpox in pediatric patients aged 2 to less than 18 years is based on single─dose pharmacokinetic and multiple─dose safety data from an open─label trial with valacyclovir and supported by efficacy and safety data from 3 randomized, double─blind, placebo─controlled trials evaluating oral acyclovir in pediatric subjects with chickenpox [see Dosage and Administration (2.2), Adverse Reactions (6.2), Clinical Pharmacology (12.3), Clinical Studies (14.4)].
The efficacy and safety of valacyclovir have not been established in pediatric patients:
- aged less than 12 years with cold sores
- aged less than 18 years with genital herpes
- aged less than 18 years with herpes zoster
- aged less than 2 years with chickenpox
- for suppressive therapy following neonatal HSV infection.
The pharmacokinetic profile and safety of valacyclovir oral suspension in children aged less than 12 years were studied in 3 open‑label trials. No efficacy evaluations were conducted in any of the 3 trials.
Trial 1 was a single‑dose pharmacokinetic, multiple‑dose safety trial in 27 pediatric subjects aged 1 to less than 12 years with clinically suspected varicella─zoster virus (VZV) infection [see Dosage and Administration (2.2), Adverse Reactions (6.2), Clinical Pharmacology (12.3), Clinical Studies (14.4)].
Trial 2 was a single‑dose pharmacokinetic and safety trial in pediatric subjects aged 1 month to less than 6 years who had an active herpes virus infection or who were at risk for herpes virus infection. Fifty─seven subjects were enrolled and received a single dose of 25 mg/kg valacyclovir oral suspension. In infants and children aged 3 months to less than 6 years, this dose provided comparable systemic acyclovir exposures to that from a 1─gram dose of valacyclovir in adults (historical data). In infants aged 1 month to less than 3 months, mean acyclovir exposures resulting from a 25─mg/kg dose were higher (Cmax: ↑30%, AUC: ↑60%) than acyclovir exposures following a 1─gram dose of valacyclovir in adults. Acyclovir is not approved for suppressive therapy in infants and children following neonatal HSV infections; therefore valacyclovir is not recommended for this indication because efficacy cannot be extrapolated from acyclovir.
Trial 3 was a single‑dose pharmacokinetic, multiple‑dose safety trial in 28 pediatric subjects aged 1 to less than 12 years with clinically suspected HSV infection. None of the subjects enrolled in this trial had genital herpes. Each subject was dosed with valacyclovir oral suspension, 10 mg/kg twice daily for 3 to 5 days. Acyclovir systemic exposures in pediatric subjects following valacyclovir oral suspension were compared with historical acyclovir systemic exposures in immunocompetent adults receiving the solid oral dosage form of valacyclovir or acyclovir for the treatment of recurrent genital herpes. The mean projected daily acyclovir systemic exposures in pediatric subjects across all age‑groups (1 to less than 12 years) were lower (Cmax: ↓20%, AUC: ↓33%) compared with the acyclovir systemic exposures in adults receiving valacyclovir 500 mg twice daily, but were higher (daily AUC: ↑16%) than systemic exposures in adults receiving acyclovir 200 mg 5 times daily. Insufficient data are available to support valacyclovir for the treatment of recurrent genital herpes in this age‑group because clinical information on recurrent genital herpes in young children is limited; therefore, extrapolating efficacy data from adults to this population is not possible. Moreover, valacyclovir has not been studied in children aged 1 to less than 12 years with recurrent genital herpes.
8.5 Geriatric Use
[Of the total number of subjects in clinical trials of VALTREX, 906 were 65 and over, and 352 were 75 and over. In a clinical trial of herpes zoster, the duration of pain after healing (post-herpetic neuralgia) was longer in subjects 65 and older compared with younger adults. Elderly patients are more likely to have reduced renal function and require dose reduction. Elderly patients are also more likely to have renal or CNS adverse events [see Dosage and Administration (2.4), Warnings and Precautions (5.2, 5.3), Clinical Pharmacology (12.3)].
10 OVERDOSAGE
Caution should be exercised to prevent inadvertent overdose [see Use in Specific Populations (8.5, 8.6)]. Precipitation of acyclovir in renal tubules may occur when the solubility (2.5 mg/mL) is exceeded in the intratubular fluid. In the event of acute renal failure and anuria, the patient may benefit from hemodialysis until renal function is restored [see Dosage and Administration (2.4)].
11 DESCRIPTION
VALTREX (valacyclovir hydrochloride) is the hydrochloride salt of the L─valyl ester of the antiviral drug acyclovir.
VALTREX Caplets are for oral administration. Each caplet contains valacyclovir hydrochloride equivalent to 500 mg or 1 gram valacyclovir and the inactive ingredients carnauba wax, colloidal silicon dioxide, crospovidone, FD&C Blue No. 2 Lake, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, povidone, and titanium dioxide. The blue, film─coated caplets are printed with edible white ink.
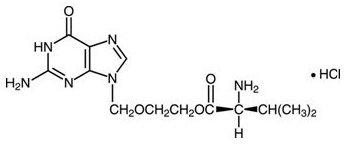
The chemical name of valacyclovir hydrochloride is L-valine, 2-[(2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy]ethyl ester, monohydrochloride. It has the following structural formula:
Valacyclovir hydrochloride is a white to off─white powder with the molecular formula C13H20N6O4HCl and a molecular weight of 360.80. The maximum solubility in water at 25°C is 174 mg/mL. The pkas for valacyclovir hydrochloride are 1.90, 7.47, and 9.43.
12 CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
The pharmacokinetics of valacyclovir and acyclovir after oral administration of VALTREX have been investigated in 14 volunteer trials involving 283 adults and in 3 trials involving 112 pediatric subjects aged 1 month to less than 12 years.
Pharmacokinetics in Adults: Absorption and Bioavailability: After oral administration, valacyclovir hydrochloride is rapidly absorbed from the gastrointestinal tract and nearly completely converted to acyclovir and L─valine by first-pass intestinal and/or hepatic metabolism.
The absolute bioavailability of acyclovir after administration of VALTREX is 54.5% ± 9.1% as determined following a 1─gram oral dose of VALTREX and a 350─mg intravenous acyclovir dose to 12 healthy volunteers. Acyclovir bioavailability from the administration of VALTREX is not altered by administration with food (30 minutes after an 873 Kcal breakfast, which included 51 grams of fat).
Acyclovir pharmacokinetic parameter estimates following administration of VALTREX to healthy adult volunteers are presented in Table 3. There was a less than dose─proportional increase in acyclovir maximum concentration (Cmax) and area under the acyclovir concentration─time curve (AUC) after single─dose and multiple─dose administration (4 times daily) of VALTREX from doses between 250 mg to 1 gram.
There is no accumulation of acyclovir after the administration of valacyclovir at the recommended dosage regimens in adults with normal renal function.
|
Dose |
Single─Dose Administration (N = 8) |
Multiple─Dose Administrationa (N = 24, 8 per treatment arm) |
||
|
Cmax (±SD) (mcg/mL) |
AUC (±SD) (hmcg/mL) |
Cmax (±SD) (mcg/mL) |
AUC (±SD) (hmcg/mL) |
|
|
100 mg |
0.83 (±0.14) |
2.28 (±0.40) |
ND |
ND |
|
250 mg |
2.15 (±0.50) |
5.76 (±0.60) |
2.11 (±0.33) |
5.66 (±1.09) |
|
500 mg |
3.28 (±0.83) |
11.59 (±1.79) |
3.69 (±0.87) |
9.88 (±2.01) |
|
750 mg |
4.17 (±1.14) |
14.11 (±3.54) |
ND |
ND |
|
1,000 mg |
5.65 (±2.37) |
19.52 (±6.04) |
4.96 (±0.64) |
15.70 (±2.27) |
|
a Administered 4 times daily for 11 days. |
||||
|
ND = not done. |
||||
Distribution: The binding of valacyclovir to human plasma proteins ranges from 13.5% to 17.9%. The binding of acyclovir to human plasma proteins ranges from 9% to 33%.
Metabolism: Valacyclovir is converted to acyclovir and L─valine by first─pass intestinal and/or hepatic metabolism. Acyclovir is converted to a small extent to inactive metabolites by aldehyde oxidase and by alcohol and aldehyde dehydrogenase. Neither valacyclovir nor acyclovir is metabolized by cytochrome P450 enzymes. Plasma concentrations of unconverted valacyclovir are low and transient, generally becoming non-quantifiable by 3 hours after administration. Peak plasma valacyclovir concentrations are generally less than 0.5 mcg/mL at all doses. After single─dose administration of 1 gram of VALTREX, average plasma valacyclovir concentrations observed were 0.5, 0.4, and 0.8 mcg/mL in subjects with hepatic dysfunction, renal insufficiency, and in healthy subjects who received concomitant cimetidine and probenecid, respectively.
Elimination: The pharmacokinetic disposition of acyclovir delivered by valacyclovir is consistent with previous experience from intravenous and oral acyclovir. Following the oral administration of a single 1 gram dose of radiolabeled valacyclovir to 4 healthy subjects, 46% and 47% of administered radioactivity was recovered in urine and feces, respectively, over 96 hours. Acyclovir accounted for 89% of the radioactivity excreted in the urine. Renal clearance of acyclovir following the administration of a single 1-gram dose of VALTREX to 12 healthy subjects was approximately 255 ± 86 mL/min which represents 42% of total acyclovir apparent plasma clearance.
The plasma elimination half‑life of acyclovir typically averaged 2.5 to 3.3 hours in all trials of VALTREX in subjects with normal renal function.
Specific Populations: Renal Impairment: Reduction in dosage is recommended in patients with renal impairment [see Dosage and Administration (2.4), Use in Specific Populations (8.5, 8.6)].
Following administration of VALTREX to subjects with ESRD, the average acyclovir half‑life is approximately 14 hours. During hemodialysis, the acyclovir half‑life is approximately 4 hours. Approximately one‑third of acyclovir in the body is removed by dialysis during a 4‑hour hemodialysis session. Apparent plasma clearance of acyclovir in subjects on dialysis was 86.3 ± 21.3 mL/min/1.73 m2 compared with 679.16 ± 162.76 mL/min/1.73 m2 in healthy subjects.
Hepatic Impairment: Administration of VALTREX to subjects with moderate (biopsy─proven cirrhosis) or severe (with and without ascites and biopsy─proven cirrhosis) liver disease indicated that the rate but not the extent of conversion of valacyclovir to acyclovir is reduced, and the acyclovir half─life is not affected. Dosage modification is not recommended for patients with cirrhosis.
HIV-1 Disease: In 9 subjects with HIV-1 disease and CD4+ cell counts less than 150 cells/mm3 who received VALTREX at a dosage of 1 gram 4 times daily for 30 days, the pharmacokinetics of valacyclovir and acyclovir were not different from that observed in healthy subjects.
Geriatrics: After single-dose administration of 1 gram of VALTREX in healthy geriatric subjects, the half‑life of acyclovir was 3.11 ± 0.51 hours, compared with 2.91 ± 0.63 hours in healthy younger adult subjects. The pharmacokinetics of acyclovir following single- and multiple‑dose oral administration of VALTREX in geriatric subjects varied with renal function. Dose reduction may be required in geriatric patients, depending on the underlying renal status of the patient [see Dosage and Administration (2.4), Use in Specific Populations (8.5. 8.6)].
Pediatrics: Acyclovir pharmacokinetics have been evaluated in a total of 98 pediatric subjects (aged 1 month to less than 12 years) following administration of the first dose of an extemporaneous oral suspension of valacyclovir [see Adverse Reactions (6.2), Use in Specific Populations (8.4)]. Acyclovir pharmacokinetic parameter estimates following a 20─mg/kg dose are provided in Table 4.
| a Historical estimates using pediatric pharmacokinetic sampling schedule. | |||||||||
|
Parameter |
Pediatric Subjects (20 mg/kg Oral Suspension) |
Adults 1─gram Solid Dose of VALTREXa (N = 15) |
|||||||
|
1 -<2 yr (N = 6) |
2 -<6 yr (N = 12) |
6 -<12 yr (N = 8) |
|||||||
|
AUC (mcgh/mL) |
14.4 (±6.26) |
10.1 (±3.35) |
13.1 (±3.43) |
17.2 (±3.10) |
|||||
|
Cmax (mcg/mL) |
4.03 (±1.37) |
3.75 (±1.14) |
4.71 (±1.20) |
4.72 (±1.37) |
|||||
Drug Interactions: When VALTREX is coadministered with antacids, cimetidine and/or probenicid, digoxin, or thiazide diuretics in patients with normal renal function, the effects are not considered to be of clinical significance (see below). Therefore, when VALTREX is coadministered with these drugs in patients with normal renal function, no dosage adjustment is recommended.
Antacids: The pharmacokinetics of acyclovir after a single dose of VALTREX (1 gram) were unchanged by coadministration of a single dose of antacids (Al3+ or Mg++).
Cimetidine: Acyclovir Cmax and AUC following a single dose of VALTREX (1 gram) increased by 8% and 32%, respectively, after a single dose of cimetidine (800 mg).
Cimetidine Plus Probenecid: Acyclovir Cmax and AUC following a single dose of VALTREX (1 gram) increased by 30% and 78%, respectively, after a combination of cimetidine and probenecid, primarily due to a reduction in renal clearance of acyclovir.
Digoxin: The pharmacokinetics of digoxin were not affected by coadministration of VALTREX 1 gram 3 times daily, and the pharmacokinetics of acyclovir after a single dose of VALTREX (1 gram) was unchanged by coadministration of digoxin (2 doses of 0.75 mg).
Probenecid: Acyclovir Cmax and AUC following a single dose of VALTREX (1 gram) increased by 22% and 49%, respectively, after probenecid (1 gram).
Thiazide Diuretics: The pharmacokinetics of acyclovir after a single dose of VALTREX (1 gram) were unchanged by coadministration of multiple doses of thiazide diuretics.
12.4 Microbiology
Mechanism of Action: Valacyclovir is a nucleoside analogue DNA polymerase inhibitor. Valacyclovir hydrochloride is rapidly converted to acyclovir which has demonstrated antiviral activity against HSV types 1 (HSV─1) and 2 (HSV─2) and VZV both in cell culture and in vivo.
The inhibitory activity of acyclovir is highly selective due to its affinity for the enzyme thymidine kinase (TK) encoded by HSV and VZV. This viral enzyme converts acyclovir into acyclovir monophosphate, a nucleotide analogue. The monophosphate is further converted into diphosphate by cellular guanylate kinase and into triphosphate by a number of cellular enzymes. In biochemical assays, acyclovir triphosphate inhibits replication of herpes viral DNA. This is accomplished in 3 ways: 1) competitive inhibition of viral DNA polymerase, 2) incorporation and termination of the growing viral DNA chain, and 3) inactivation of the viral DNA polymerase. The greater antiviral activity of acyclovir against HSV compared with VZV is due to its more efficient phosphorylation by the viral TK.
Antiviral Activities: The quantitative relationship between the cell culture susceptibility of herpesviruses to antivirals and the clinical response to therapy has not been established in humans, and virus sensitivity testing has not been standardized. Sensitivity testing results, expressed as the concentration of drug required to inhibit by 50% the growth of virus in cell culture (EC50), vary greatly depending upon a number of factors. Using plaque-reduction assays, the EC50 values against herpes simplex virus isolates range from 0.09 to 60 μM (0.02 to 13.5 mcg/mL) for HSV─1 and from 0.04 to 44 µM (0.01 to 9.9 mcg/mL) for HSV─2. The EC50 values for acyclovir against most laboratory strains and clinical isolates of VZV range from 0.53 to 48 µM (0.12 to 10.8 mcg/mL). Acyclovir also demonstrates activity against the Oka vaccine strain of VZV with a mean EC50 of 6 µM (1.35 mcg/mL).
Resistance: Resistance of HSV and VZV to acyclovir can result from qualitative and quantitative changes in the viral TK and/or DNA polymerase. Clinical isolates of VZV with reduced susceptibility to acyclovir have been recovered from patients with AIDS. In these cases, TK-deficient mutants of VZV have been recovered.
Resistance of HSV and VZV to acyclovir occurs by the same mechanisms. While most of the acyclovir─resistant mutants isolated thus far from immunocompromised patients have been found to be TK─deficient mutants, other mutants involving the viral TK gene (TK partial and TK altered) and DNA polymerase have also been isolated. TK─negative mutants may cause severe disease in immunocompromised patients. The possibility of viral resistance to valacyclovir (and therefore, to acyclovir) should be considered in patients who show poor clinical response during therapy.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
The data presented below include references to the steady─state acyclovir AUC observed in humans treated with 1 gram VALTREX given orally 3 times a day to treat herpes zoster. Plasma drug concentrations in animal studies are expressed as multiples of human exposure to acyclovir [see Clinical Pharmacology (12.3)].
Valacyclovir was noncarcinogenic in lifetime carcinogenicity bioassays at single daily doses (gavage) of valacyclovir giving plasma acyclovir concentrations equivalent to human levels in the mouse bioassay and 1.4 to 2.3 times human levels in the rat bioassay. There was no significant difference in the incidence of tumors between treated and control animals, nor did valacyclovir shorten the latency of tumors.
Valacyclovir was tested in 5 genetic toxicity assays. An Ames assay was negative in the absence or presence of metabolic activation. Also negative were an in vitro cytogenetic study with human lymphocytes and a rat cytogenetic study.
In the mouse lymphoma assay, valacyclovir was not mutagenic in the absence of metabolic activation. In the presence of metabolic activation (76% to 88% conversion to acyclovir), valacyclovir was mutagenic.
Valacyclovir was mutagenic in a mouse micronucleus assay.
Valacyclovir did not impair fertility or reproduction in rats at 6 times human plasma levels.
14 CLINICAL STUDIES
14.1 Cold Sores (Herpes Labialis)
Two double‑blind, placebo‑controlled clinical trials were conducted in 1,856 healthy adults and adolescents (aged greater than or equal to 12 years) with a history of recurrent cold sores. Subjects self‑initiated therapy at the earliest symptoms and prior to any signs of a cold sore. The majority of subjects initiated treatment within 2 hours of onset of symptoms. Subjects were randomized to VALTREX 2 grams twice daily on Day 1 followed by placebo on Day 2, VALTREX 2 grams twice daily on Day 1 followed by 1 gram twice daily on Day 2, or placebo on Days 1 and 2.
The mean duration of cold sore episodes was about 1 day shorter in treated subjects as compared with placebo. The 2─day regimen did not offer additional benefit over the 1─day regimen.
No significant difference was observed between subjects receiving VALTREX or placebo in the prevention of progression of cold sore lesions beyond the papular stage.
14.2 Genital Herpes Infections
Initial Episode: Six hundred forty─three immunocompetent adults with first─episode genital herpes who presented within 72 hours of symptom onset were randomized in a double─blind trial to receive 10 days of VALTREX 1 gram twice daily (n = 323) or oral acyclovir 200 mg 5 times a day (n = 320). For both treatment groups the median time to lesion healing was 9 days, the median time to cessation of pain was 5 days, and the median time to cessation of viral shedding was 3 days.
Recurrent Episodes: Three double─blind trials (2 of them placebo─controlled) in immunocompetent adults with recurrent genital herpes were conducted. Subjects self─initiated therapy within 24 hours of the first sign or symptom of a recurrent genital herpes episode.
In 1 trial, subjects were randomized to receive 5 days of treatment with either VALTREX 500 mg twice daily (n = 360) or placebo (n = 259). The median time to lesion healing was 4 days in the group receiving VALTREX 500 mg versus 6 days in the placebo group, and the median time to cessation of viral shedding in subjects with at least 1 positive culture (42% of the overall trial population) was 2 days in the group receiving VALTREX 500 mg versus 4 days in the placebo group. The median time to cessation of pain was 3 days in the group receiving VALTREX 500 mg versus 4 days in the placebo group. Results supporting efficacy were replicated in a second trial.
In a third trial, subjects were randomized to receive VALTREX 500 mg twice daily for 5 days (n = 398) or VALTREX 500 mg twice daily for 3 days (and matching placebo twice daily for 2 additional days) (n = 402). The median time to lesion healing was about 4½ days in both treatment groups. The median time to cessation of pain was about 3 days in both treatment groups.
Suppressive Therapy: Two clinical trials were conducted, one in immunocompetent adults and one in HIV-1─infected adults.
A double‑blind, 12‑month, placebo‑ and active‑controlled trial enrolled immunocompetent adults with a history of 6 or more recurrences per year. Outcomes for the overall trial population are shown in Table 5.
| a Includes lost to follow-up, discontinuations due to adverse events, and consent withdrawn. | ||||||
|
Outcome |
6 Months |
12 Months |
||||
|
VALTREX 1 gram Once Daily (n = 269) |
Oral Acyclovir 400 mg Twice Daily (n = 267) |
Placebo (n = 134) |
VALTREX 1 gram Once Daily (n = 269) |
Oral Acyclovir 400 mg Twice Daily (n = 267) |
Placebo (n = 134) |
|
|
Recurrence free |
55% |
54% |
7% |
34% |
34% |
4% |
|
Recurrences |
35% |
36% |
83% |
46% |
46% |
85% |
|
Unknowna |
10% |
10% |
10% |
19% |
19% |
10% |
Subjects with 9 or fewer recurrences per year showed comparable results with VALTREX 500 mg once daily.
In a second trial, 293 HIV‑1-infected adults on stable antiretroviral therapy with a history of 4 or more recurrences of ano‑genital herpes per year were randomized to receive either VALTREX 500 mg twice daily (n = 194) or matching placebo (n = 99) for 6 months. The median duration of recurrent genital herpes in enrolled subjects was 8 years, and the median number of recurrences in the year prior to enrollment was 5. Overall, the median pretrial HIV‑1 RNA was 2.6 log10 copies/mL. Among subjects who received VALTREX, the pretrial median CD4+ cell count was 336 cells/mm3; 11% had less than 100 cells/mm3, 16% had 100 to 199 cells/mm3, 42% had 200 to 499 cells/mm3, and 31% had greater than or equal to 500 cells/mm3. Outcomes for the overall trial population are shown in Table 6.
| a Includes lost to follow-up, discontinuations due to adverse events, and consent withdrawn. | |||||||||
|
Outcome |
VALTREX 500 mg Twice Daily (n = 194) |
Placebo (n = 99) |
|||||||
|
Recurrence free |
65% |
26% |
|||||||
|
Recurrences |
17% |
57% |
|||||||
|
Unknowna |
18% |
17% |
|||||||
Reduction of Transmission of Genital Herpes: A double─blind, placebo─controlled trial to assess transmission of genital herpes was conducted in 1,484 monogamous, heterosexual, immunocompetent adult couples. The couples were discordant for HSV─2 infection. The source partner had a history of 9 or fewer genital herpes episodes per year. Both partners were counseled on safer sex practices and were advised to use condoms throughout the trial period. Source partners were randomized to treatment with either VALTREX 500 mg once daily or placebo once daily for 8 months. The primary efficacy endpoint was symptomatic acquisition of HSV─2 in susceptible partners. Overall HSV─2 acquisition was defined as symptomatic HSV─2 acquisition and/or HSV─2 seroconversion in susceptible partners. The efficacy results are summarized in Table 7.
| a Results show reductions in risk of 75% (symptomatic HSV─2 acquisition), 50% (HSV─2 seroconversion), and 48% (overall HSV─2 acquisition) with VALTREX versus placebo. Individual results may vary based on consistency of safer sex practices. | ||||||||||||
|
Endpoint |
VALTREXa (n = 743) |
Placebo (n = 741) |
||||||||||
|
Symptomatic HSV─2 acquisition |
4 (0.5%) |
16 (2.2%) |
||||||||||
|
HSV─2 seroconversion |
12 (1.6%) |
24 (3.2%) |
||||||||||
|
Overall HSV─2 acquisition |
14 (1.9%) |
27 (3.6%) |
||||||||||
14.3 Herpes Zoster
Two randomized double‑blind clinical trials in immunocompetent adults with localized herpes zoster were conducted. VALTREX was compared with placebo in subjects aged less than 50 years, and with oral acyclovir in subjects aged greater than 50 years. All subjects were treated within 72 hours of appearance of zoster rash. In subjects aged less than 50 years, the median time to cessation of new lesion formation was 2 days for those treated with VALTREX compared with 3 days for those treated with placebo. In subjects aged greater than 50 years, the median time to cessation of new lesions was 3 days in subjects treated with either VALTREX or oral acyclovir. In subjects aged less than 50 years, no difference was found with respect to the duration of pain after healing (post‑herpetic neuralgia) between the recipients of VALTREX and placebo. In subjects aged greater than 50 years, among the 83% who reported pain after healing (post‑herpetic neuralgia), the median duration of pain after healing [95% confidence interval] in days was: 40 [31, 51], 43 [36, 55], and 59 [41, 77] for 7‑day VALTREX, 14‑day VALTREX, and 7‑day oral acyclovir, respectively.
14.4 Chickenpox
The use of VALTREX for treatment of chickenpox in pediatric subjects aged 2 to less than 18 years is based on single‑dose pharmacokinetic and multiple‑dose safety data from an open‑label trial with valacyclovir and supported by safety and extrapolated efficacy data from 3 randomized, double‑blind, placebo‑controlled trials evaluating oral acyclovir in pediatric subjects.
The single‑dose pharmacokinetic and multiple‑dose safety trial enrolled 27 pediatric subjects aged 1 to less than 12 years with clinically suspected VZV infection. Each subject was dosed with valacyclovir oral suspension, 20 mg/kg 3 times daily for 5 days. Acyclovir systemic exposures in pediatric subjects following valacyclovir oral suspension were compared with historical acyclovir systemic exposures in immunocompetent adults receiving the solid oral dosage form of valacyclovir or acyclovir for the treatment of herpes zoster. The mean projected daily acyclovir exposures in pediatric subjects across all age‑groups (1 to less than 12 years) were lower (Cmax: ↓13%, AUC: ↓30%) than the mean daily historical exposures in adults receiving valacyclovir 1 gram 3 times daily, but were higher (daily AUC: ↑50%) than the mean daily historical exposures in adults receiving acyclovir 800 mg 5 times daily. The projected daily exposures in pediatric subjects were greater (daily AUC approximately 100% greater) than the exposures seen in immunocompetent pediatric subjects receiving acyclovir 20 mg/kg 4 times daily for the treatment of chickenpox. Based on the pharmacokinetic and safety data from this trial and the safety and extrapolated efficacy data from the acyclovir trials, oral valacyclovir 20 mg/kg 3 times a day for 5 days (not to exceed 1 gram 3 times daily) is recommended for the treatment of chickenpox in pediatric patients aged 2 to less than 18 years. Because the efficacy and safety of acyclovir for the treatment of chickenpox in children aged less than 2 years have not been established, efficacy data cannot be extrapolated to support valacyclovir treatment in children aged less than 2 years with chickenpox. Valacyclovir is also not recommended for the treatment of herpes zoster in children because safety data up to 7 days’ duration are not available [see Use in Specific Populations (8.4)].
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA‑Approved Patient Labeling (Patient Information).
Importance of Adequate Hydration: Patients should be advised to maintain adequate hydration.
Cold Sores (Herpes Labialis): Patients should be advised to initiate treatment at the earliest symptom of a cold sore (e.g., tingling, itching, or burning). There are no data on the effectiveness of treatment initiated after the development of clinical signs of a cold sore (e.g., papule, vesicle, or ulcer). Patients should be instructed that treatment for cold sores should not exceed 1 day (2 doses) and that their doses should be taken about 12 hours apart. Patients should be informed that VALTREX is not a cure for cold sores.
Genital Herpes: Patients should be informed that VALTREX is not a cure for genital herpes. Because genital herpes is a sexually transmitted disease, patients should avoid contact with lesions or intercourse when lesions and/or symptoms are present to avoid infecting partners. Genital herpes is frequently transmitted in the absence of symptoms through asymptomatic viral shedding. Therefore, patients should be counseled to use safer sex practices in combination with suppressive therapy with VALTREX. Sex partners of infected persons should be advised that they might be infected even if they have no symptoms. Type‑specific serologic testing of asymptomatic partners of persons with genital herpes can determine whether risk for HSV‑2 acquisition exists.
VALTREX has not been shown to reduce transmission of sexually transmitted infections other than HSV‑2.
If medical management of a genital herpes recurrence is indicated, patients should be advised to initiate therapy at the first sign or symptom of an episode.
There are no data on the effectiveness of treatment initiated more than 72 hours after the onset of signs and symptoms of a first episode of genital herpes or more than 24 hours after the onset of signs and symptoms of a recurrent episode.
There are no data on the safety or effectiveness of chronic suppressive therapy of more than 1 year’s duration in otherwise healthy patients. There are no data on the safety or effectiveness of chronic suppressive therapy of more than 6 months’ duration in HIV‑1-infected patients.
Herpes Zoster: There are no data on treatment initiated more than 72 hours after onset of the zoster rash. Patients should be advised to initiate treatment as soon as possible after a diagnosis of herpes zoster.
Chickenpox: Patients should be advised to initiate treatment at the earliest sign or symptom of chickenpox.
VALTREX is a registered trademark of the GlaxoSmithKline group of companies.
Distributed by:
GlaxoSmithKline
Research Triangle Park, NC 27709
©2013, GlaxoSmithKline group of companies. All rights reserved.
VTX:6PI
PHARMACIST‑DETACH HERE AND GIVE INSTRUCTIONS TO PATIENT
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
Patient Information
VALTREX® (VAL-trex)
(valacyclovir hydrochloride) Caplets
Read the Patient Information that comes with VALTREX before you start using it and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment. Ask your healthcare provider or pharmacist if you have questions.
What is VALTREX?
VALTREX is a prescription antiviral medicine. VALTREX lowers the ability of herpes viruses to multiply in your body.
VALTREX is used in adults:
- to treat cold sores (also called fever blisters or herpes labialis)
- to treat shingles (also called herpes zoster)
- to treat or control genital herpes outbreaks in adults with normal immune systems
- to control genital herpes outbreaks in adults infected with the human immunodeficiency virus (HIV-1) with CD4+ cell count greater than 100 cells/mm3
- with safer sex practices to lower the chances of spreading genital herpes to others. Even with safer sex practices, it is still possible to spread genital herpes.
VALTREX used daily with the following safer sex practices can lower the chances of passing genital herpes to your partner.
- Do not have sexual contact with your partner when you have any symptom or outbreak of genital herpes.
- Use a condom made of latex or polyurethane whenever you have sexual contact.
VALTREX is used in children:
- to treat cold sores (for children aged greater than or equal to 12 years)
- to treat chickenpox (for children aged 2 to less than 18 years).
VALTREX does not cure herpes infections (cold sores, chickenpox, shingles, or genital herpes).
The efficacy of VALTREX has not been studied in children who have not reached puberty.
What are cold sores, chickenpox, shingles, and genital herpes?
Cold sores are caused by a herpes virus that may be spread by kissing or other physical contact with the infected area of the skin. They are small, painful ulcers that you get in or around your mouth. It is not known if VALTREX can stop the spread of cold sores to others.
Chickenpox is caused by a herpes virus. It causes an itchy rash of multiple small, red bumps that look like pimples or insect bites usually appearing first on the abdomen or back and face. It can spread to almost everywhere else on the body and may be accompanied by flu-like symptoms.
Shingles is caused by the same herpes virus that causes chickenpox. It causes small, painful blisters that happen on your skin. Shingles occurs in people who have already had chickenpox. Shingles can be spread to people who have not had chickenpox or the chickenpox vaccine by contact with the infected areas of the skin. It is not known if VALTREX can stop the spread of shingles to others.
Genital herpes is a sexually transmitted disease. It causes small, painful blisters on your genital area. You can spread genital herpes to others, even when you have no symptoms. If you are sexually active, you can still pass herpes to your partner, even if you are taking VALTREX. VALTREX, taken every day as prescribed and used with the following safer sex practices, can lower the chances of passing genital herpes to your partner.
- Do not have sexual contact with your partner when you have any symptom or outbreak of genital herpes.
- Use a condom made of latex or polyurethane whenever you have sexual contact.
Ask your healthcare provider for more information about safer sex practices.
Who should not take VALTREX?
Do not take VALTREX if you are allergic to any of its ingredients or to acyclovir. The active ingredient is valacyclovir. See the end of this leaflet for a complete list of ingredients in VALTREX.
Before taking VALTREX, tell your healthcare provider:
About all of your medical conditions, including:
- if you have had a bone marrow transplant or kidney transplant, or if you have advanced HIV-1 disease or "AIDS". Patients with these conditions may have a higher chance for getting a blood disorder called thrombotic thrombocytopenic purpura/hemolytic uremic syndrome (TTP/HUS). TTP/HUS can result in death.
- if you have kidney problems. Patients with kidney problems may have a higher chance for getting side effects or more kidney problems with VALTREX. Your healthcare provider may give you a lower dose of VALTREX.
- if you are aged 65 years or older. Elderly patients have a higher chance of certain side effects. Also, elderly patients are more likely to have kidney problems. Your healthcare provider may give you a lower dose of VALTREX.
- if you are pregnant or planning to become pregnant. Talk with your healthcare provider about the risks and benefits of taking prescription drugs (including VALTREX) during pregnancy.
- if you are breastfeeding. VALTREX may pass into your milk and it may harm your baby. Talk with your healthcare provider about the best way to feed your baby if you are taking VALTREX.
- about all the medicines you take, including prescription and non─prescription medicines, vitamins, and herbal supplements. VALTREX may affect other medicines, and other medicines may affect VALTREX. It is a good idea to keep a complete list of all the medicines you take. Show this list to your healthcare provider and pharmacist any time you get a new medicine.
How should I take VALTREX?
Take VALTREX exactly as prescribed by your healthcare provider. Your dose of VALTREX and length of treatment will depend on the type of herpes infection that you have and any other medical problems that you have.
- Do not stop VALTREX or change your treatment without talking to your healthcare provider.
- VALTREX can be taken with or without food.
- If you are taking VALTREX to treat cold sores, chickenpox, shingles, or genital herpes, you should start treatment as soon as possible after your symptoms start. VALTREX may not help you if you start treatment too late.
- If you miss a dose of VALTREX, take it as soon as you remember and then take your next dose at its regular time. However, if it is almost time for your next dose, do not take the missed dose. Wait and take the next dose at the regular time.
- Do not take more than the prescribed number of VALTREX Caplets each day. Call your healthcare provider right away if you take too much VALTREX.
What are the possible side effects of VALTREX?
Kidney failure and nervous system problems are not common, but can be serious in some patients taking VALTREX. Nervous system problems include aggressive behavior, unsteady movement, shaky movements, confusion, speech problems, hallucinations (seeing or hearing things that are really not there), seizures, and coma. Kidney failure and nervous system problems have happened in patients who already have kidney disease and in elderly patients whose kidneys do not work well due to age. Always tell your healthcare provider if you have kidney problems before taking VALTREX. Call your doctor right away if you get a nervous system problem while you are taking VALTREX.
Common side effects of VALTREX in adults include headache, nausea, stomach pain, vomiting, and dizziness. Side effects in HIV-1-infected adults include headache, tiredness, and rash. These side effects usually are mild and do not cause patients to stop taking VALTREX.
Other less common side effects in adults include painful periods in women, joint pain, depression, low blood cell counts, and changes in tests that measure how well the liver and kidneys work.
The most common side effect seen in children aged less than 18 years was headache.
Talk to your healthcare provider if you develop any side effects that concern you.
These are not all the side effects of VALTREX. For more information ask your healthcare provider or pharmacist.
How should I store VALTREX?
- Store VALTREX Caplets at room temperature, 59° to 77°F (15° to 25°C).
- Store VALTREX suspension between 2° to 8°C (36° to 46°F) in a refrigerator. Discard after 28 days.
- Keep VALTREX in a tightly closed container.
- Do not keep medicine that is out of date or that you no longer need.
- Keep VALTREX and all medicines out of the reach of children.
General information about VALTREX
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use VALTREX for a condition for which it was not prescribed. Do not give VALTREX to other people, even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about VALTREX. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about VALTREX that is written for health professionals. More information is available at www.VALTREX.com.
What are the ingredients in VALTREX?
Active Ingredient: valacyclovir hydrochloride
Inactive Ingredients: carnauba wax, colloidal silicon dioxide, crospovidone, FD&C Blue No. 2 Lake, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, povidone, and titanium dioxide.
VALTREX is a registered trademark of the GlaxoSmithKline group of companies.
Distributed by:
GlaxoSmithKline
Research Triangle Park, NC 27709
©2013, GlaxoSmithKline group of companies. All rights reserved.
November 2013
VTX:5PIL
| VALTREX
valacyclovir hydrochloride tablet, film coated |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| VALTREX
valacyclovir hydrochloride tablet, film coated |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - A-S Medication Solutions (830016429) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| A-S Medication Solutions | 830016429 | RELABEL(50090-0557, 50090-0753) , REPACK(50090-0557) | |