OsmoFlex by Sarati International, Inc. OSMOFLEX- menthol cream
OsmoFlex by
Drug Labeling and Warnings
OsmoFlex by is a Otc medication manufactured, distributed, or labeled by Sarati International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
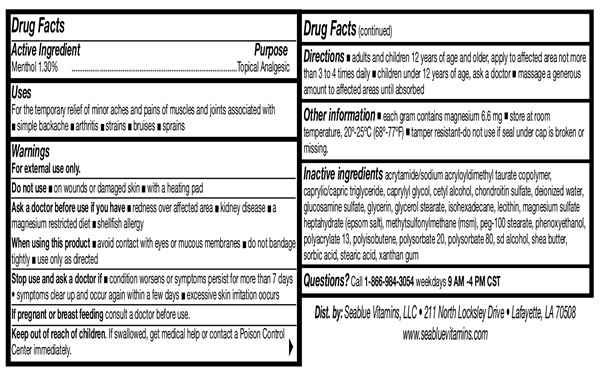
- Drug FactsActive Ingredient
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop Use and Ask A Doctor
- Keep out of reach of children
- Directions
-
Inactive Ingredients
acrylamide/sodium acryloyldimethyl taurate copolymer, caprylic/capric triglyceride, caprylyl glycol, cetyl alcohol, chondroitin sulfate, deionized water, glucosamine sulfate, glycerin, glycerol stearate, isohexadecane, lecithin, magnesium sulfate heptahydrate (epsom salt), methylsulfonylmethane (msm), peg-100, stearate, phenoxyethanol, polyacrylate 13, polyisobutene, polysorbate 20, polysorbate 80, sd alcohol, shea butter, sorbic acid, stearic acid, xanthan gum
- Questions
- Other Information
- OsmoFlex product label
-
INGREDIENTS AND APPEARANCE
OSMOFLEX
menthol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 67676-101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1.3 g in 100 g Inactive Ingredients Ingredient Name Strength ACRYLAMIDE (UNII: 20R035KLCI) TRICAPRYLIN (UNII: 6P92858988) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CETYL ALCOHOL (UNII: 936JST6JCN) CHONDROITIN SULFATE (BOVINE) (UNII: 6IC1M3OG5Z) WATER (UNII: 059QF0KO0R) GLUCOSAMINE SULFATE (UNII: 1FW7WLR731) GLYCERYL STEARATE CITRATE (UNII: WH8T92A065) ISOHEXADECANE (UNII: 918X1OUF1E) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) MAGNESIUM SULFATE HEPTAHYDRATE (UNII: SK47B8698T) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) POLYOXYL 100 STEARATE (UNII: YD01N1999R) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYISOBUTYLENE (1200000 MW) (UNII: FLT10CH37X) POLYSORBATE 20 (UNII: 7T1F30V5YH) POLYSORBATE 80 (UNII: 6OZP39ZG8H) ALCOHOL (UNII: 3K9958V90M) SHEA BUTTER (UNII: K49155WL9Y) SORBIC ACID (UNII: X045WJ989B) STEARIC ACID (UNII: 4ELV7Z65AP) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67676-101-01 113.4 g in 1 TUBE; Type 0: Not a Combination Product 01/01/1993 2 NDC: 67676-101-04 453.6 g in 1 BOTTLE; Type 0: Not a Combination Product 05/16/2016 3 NDC: 67676-101-03 964.3 g in 1 BOTTLE; Type 0: Not a Combination Product 05/16/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 01/01/1993 Labeler - Sarati International, Inc. (160219770) Registrant - Sarati International, Inc. (160219770) Establishment Name Address ID/FEI Business Operations Sarati International, Inc. 160219770 manufacture(67676-101)
Trademark Results [OsmoFlex]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 OSMOFLEX 85404035 4121867 Live/Registered |
SeaBlue Vitamins, LLC 2011-08-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.