ADVANTUS SOFT CHEW- imidacloprid tablet, chewable
Advantus by
Drug Labeling and Warnings
Advantus by is a Animal medication manufactured, distributed, or labeled by Bayer HealthCare, LLC Animal Health Division. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active Ingredients:
- Use:
- Warnings:
- Side Effects:
-
Directions:
You should weigh your dog before giving this medication to make sure you are using the right size for your dog. Do not give to puppies younger than 10 weeks of age or to dogs weighing less than 4 pounds. Do not give more than one tablet a day.
Use the table to find the right dose for your dog:
Dosing Table Body Weight
Dose
Soft Chew Strength
4-22 lbs
One soft chew
7.5 mg imidacloprid
23-110 lbs
One soft chew
37.5 mg imidacloprid
To give advantus™, place the soft chew directly in your dog’s mouth or allow your dog to take the soft chew from your hand. Watch closely to ensure your dog swallows the soft chew.
A single dose kills adult fleas on your dog. Because advantus™ does not have an effect on fleas in the environment, your dog may become re-infested with fleas. If your dog becomes reinfested, you can safely repeat treatment as often as once a day. Contact your veterinarian if you have any questions about flea infestations on your dog.
Treat all pets in your household with a flea protection product. advantus™ should only be given to dogs.
-
SPL UNCLASSIFIED SECTION
Questions/Comments? Call Bayer at 1-800-255-6826. To report side effects, call Bayer Veterinary Services at 1-800-422-9874. For additional information for reporting side effects for animal drugs, contact FDA at 1-888-FDA-VETS or online at http://www.fda.gov/AnimalVeterinary/SafetyHealth.
-
Other Information:
- Contact your veterinarian if your dog ingests more than the recommended dose of advantus™.
- advantus™ has not been tested in pregnant or nursing dogs.
- advantus™ starts to kill fleas within 1 hour. A study demonstrated that advantus™ achieves greater than 96% effectiveness against adult fleas within 4 hours.
- advantus™ may be used together with other products, including heartworm preventatives, corticosteroids, antibiotics, vaccines, deworming medications and shampoos.
- advantus™ does not protect against ticks or mosquitoes.
Flea Infestations on Dogs:
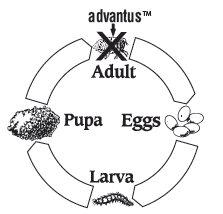
Treatment of flea infestations is important to your dog’s health. Fleas in your dog’s environment can re-infest your dog. Therefore, you may need to treat with advantus™ more than one time. The following diagram illustrates the flea’s life cycle and where advantus™ works:
Fleas reproduce rapidly. A single female flea may produce up to 2,000 eggs over her lifetime. Eggs hatch and may grow into adults in only three weeks. Adult female fleas feed by ingesting blood from your dog and lay eggs that drop off from your dog’s coat. Within days, larvae hatch from these eggs and live undetected in your dog’s surroundings, such as in the carpet and bedding. Fleas can emerge to re-infest your pet, continuing the life cycle. When these new fleas are seen on your dog, treat with advantus™.
If you have any questions about flea treatment or medical problems associated with flea infestations, consult your veterinarian.
- Storage Information:
-
How Supplied:
advantus™ is available in two strengths: 7.5 mg or 37.5 mg imidacloprid. Both strengths are packaged in 7 count bottles containing 7 flavored soft chews or 30 count bottles containing 30 flavored soft chews.
NADA 141-435, Approved by FDA
Bayer HealthCare LLC, Animal Health Division, Shawnee Mission, KS 66201 USA
©2016 Bayer 85694731 LV1702
Bayer (reg’d), the Bayer Cross (reg’d) and advantusTM are trademarks of Bayer.
Made in Spain.
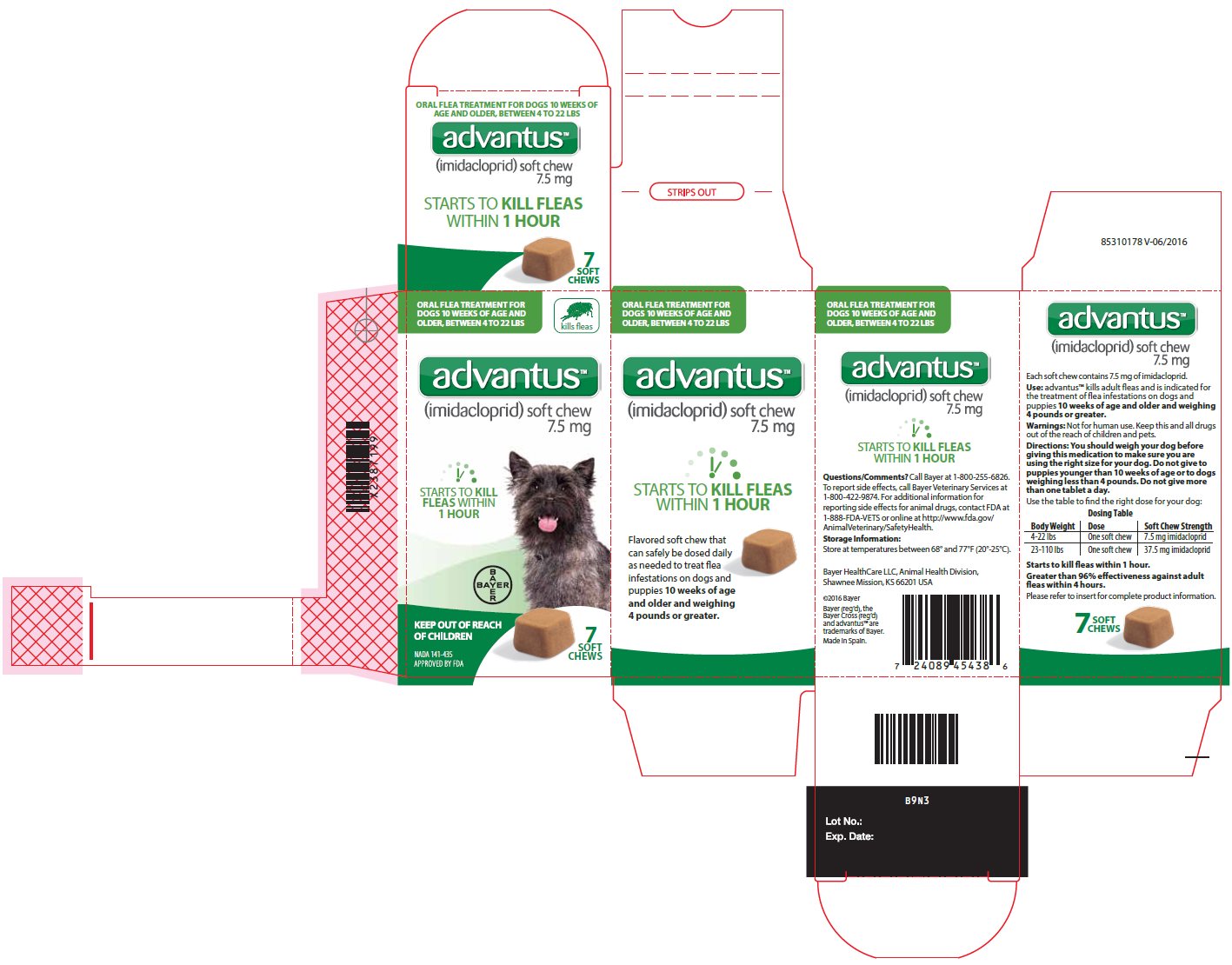
- Principal Display Panel – 7.5 mg carton label
-
Principal Display Panel – 7.5 mg unit label
ORAL FLEA TREATMENT FOR
DOGS 10 WEEKS OF AGE AND OLDER,
BETWEEN 4 TO 22 LBSadvantusTM
(imidacloprid) soft chew
7.5 mgSTARTS TO KILL FLEAS WITHIN 1 HOUR
KEEP OUT OF REACH OF CHILDREN
NADA 141-435
APPROVED BY FDA7 SOFT CHEWS
ORAL FLEA TREATMENT FOR
DOGS 10 WEEKS OF AGE AND
OLDER, BETWEEN 4 TO 22 LBSkills fleas
advantusTM
(imidacloprid) soft chew
7.5 mgSTARTS TO KILL FLEAS WITHIN 1 HOUR
KEEP OUT OF REACH OF CHILDREN
NADA 141-435
APPROVED BY FDA30 SOFT CHEWS
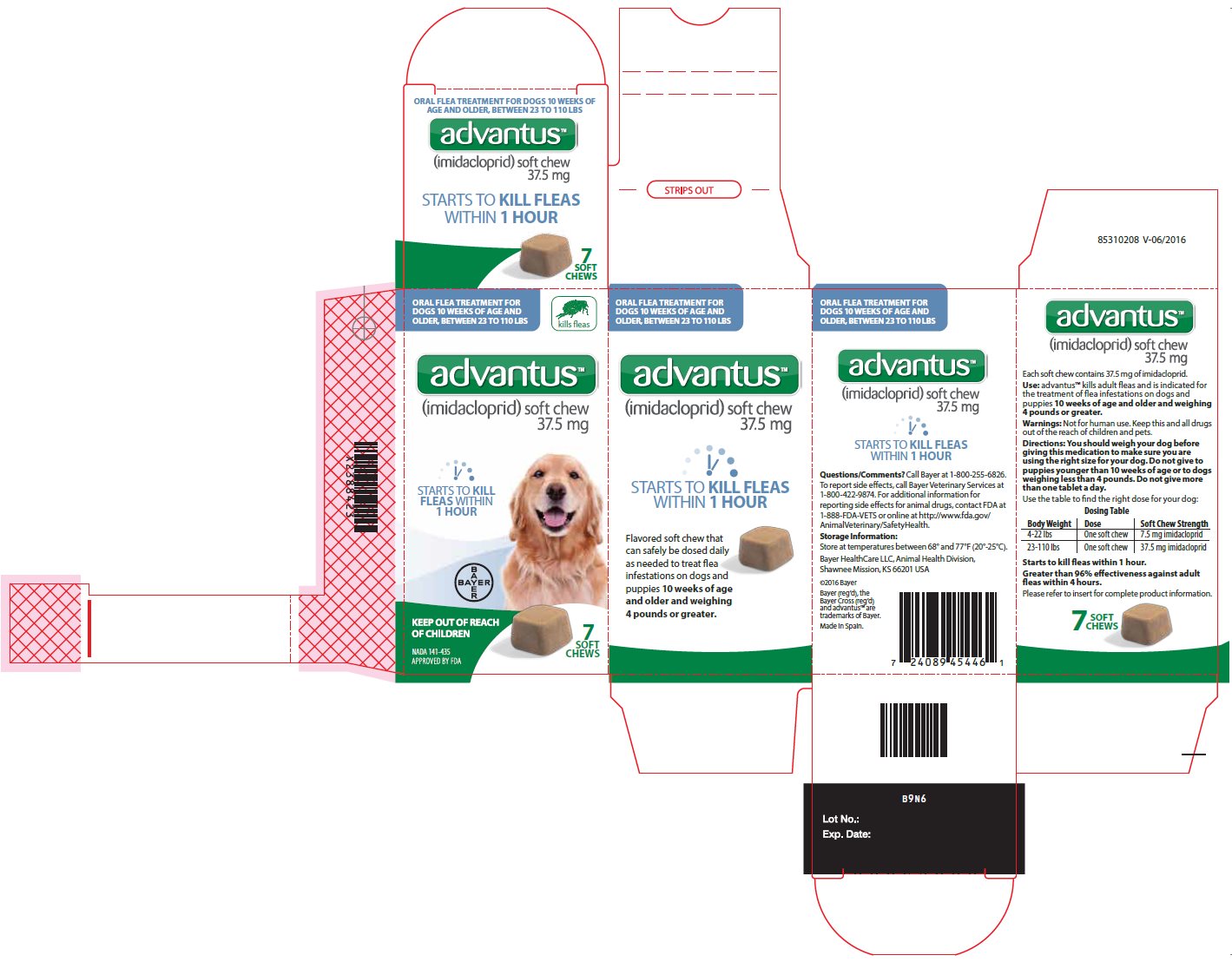
- Principal Display Panel – 37.5 mg carton label
-
Principal Display Panel – 37.5 mg unit label
ORAL FLEA TREATMENT FOR
DOGS 10 WEEKS OF AGE AND OLDER,
BETWEEN 23 TO 110 LBSkills fleas
advantusTM
(imidacloprid) soft chew
37.5 mgSTARTS TO KILL FLEAS WITHIN 1 HOUR
KEEP OUT OF REACH OF CHILDREN
NADA 141-435, APPROVED BY FDA
7 SOFT CHEWS
ORAL FLEA TREATMENT FOR
DOGS 10 WEEKS OF AGE AND OLDER,
BETWEEN 23 TO 110 LBSkills fleas
advantusTM
(imidacloprid) soft chew
37.5 mgSTARTS TO KILL FLEAS WITHIN 1 HOUR
KEEP OUT OF REACH OF CHILDREN
NADA 141-435
APPROVED BY FDA30 SOFT CHEWS
-
INGREDIENTS AND APPEARANCE
ADVANTUS SOFT CHEW
imidacloprid tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 0859-2404 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IMIDACLOPRID (UNII: 3BN7M937V8) (IMIDACLOPRID - UNII:3BN7M937V8) IMIDACLOPRID 7.5 mg Product Characteristics Color RED Score no score Shape TRAPEZOID Size 14mm Flavor MEAT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0859-2404-02 1 in 1 CARTON 1 NDC: 0859-2404-01 7 in 1 BOTTLE 2 NDC: 0859-2404-03 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141435 01/01/2017 ADVANTUS SOFT CHEW
imidacloprid tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 0859-2405 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IMIDACLOPRID (UNII: 3BN7M937V8) (IMIDACLOPRID - UNII:3BN7M937V8) IMIDACLOPRID 37.5 mg Product Characteristics Color BROWN Score no score Shape TRAPEZOID Size 14mm Flavor MEAT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0859-2405-02 1 in 1 CARTON 1 NDC: 0859-2405-01 7 in 1 BOTTLE 2 NDC: 0859-2405-03 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141435 01/01/2017 Labeler - Bayer HealthCare, LLC Animal Health Division (152266193)
Trademark Results [Advantus]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ADVANTUS 87427503 5291174 Live/Registered |
Advantus, Corp. 2017-04-27 |
 ADVANTUS 87427441 5291173 Live/Registered |
Advantus, Corp. 2017-04-27 |
 ADVANTUS 87427396 5291172 Live/Registered |
Advantus, Corp. 2017-04-27 |
 ADVANTUS 87344786 5290412 Live/Registered |
Advantus, Corp. 2017-02-22 |
 ADVANTUS 87233974 5331239 Live/Registered |
Bayer HealthCare LLC 2016-11-11 |
 ADVANTUS 85938558 not registered Dead/Abandoned |
Bayer HealthCare LLC 2013-05-21 |
 ADVANTUS 85476135 4552147 Live/Registered |
Camus Hydronics Limited 2011-11-18 |
 ADVANTUS 78793112 not registered Dead/Abandoned |
AETNA INC. 2006-01-17 |
 ADVANTUS 78793045 not registered Dead/Abandoned |
AETNA INC. 2006-01-17 |
 ADVANTUS 74575374 2663467 Live/Registered |
ADVANTUS CAPITAL MANAGEMENT, INC. 1994-09-19 |
 ADVANTUS 74257569 not registered Dead/Abandoned |
Anthem Health Systems, Inc. 1992-03-20 |
 ADVANTUS 74250338 1834294 Dead/Cancelled |
AMERICAN CYANAMID COMPANY 1992-02-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.