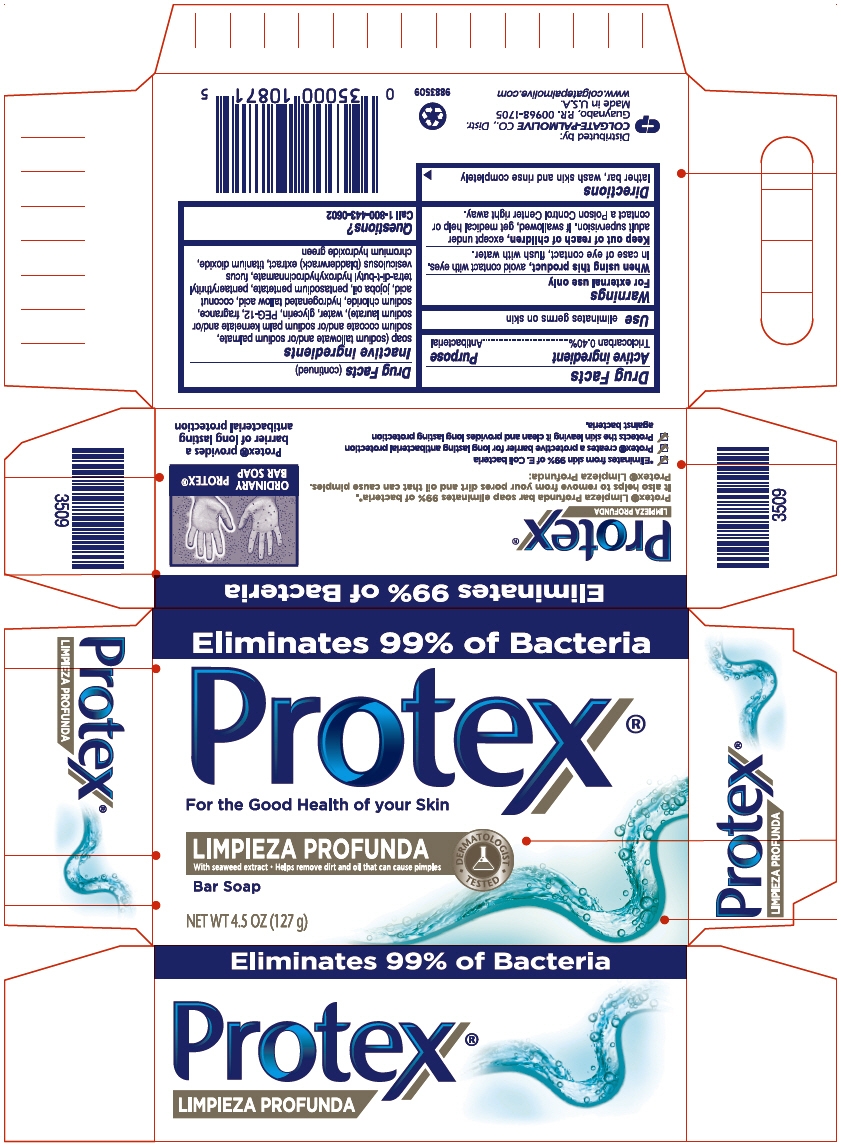
PROTEX DEEP CLEAN- triclocarban soap
Colgate-Palmolive Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Triclocarban 0.40%
Use
eliminates germs on skin
Warnings
For external use only
When using this product, avoid contact with eyes. In case of eye contact, flush with water.
Keep out of reach of children, except under adult supervision. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
lather bar, wash skin and rinse completely
Inactive ingredients
soap (sodium tallowate and/or sodium palmate, sodium cocoate and/or sodium palm kernelate and/or sodium laurate), water, glycerin, PEG-12, fragrance, sodium chloride, hydrogenated tallow acid, coconut acid, jojoba oil, pentasodium pentetate, pentaerythrityl tetra-di-t-butyl hydroxyhydrocinnamate, fucus vesiculosus (bladderwrack) extract, titanium dioxide, chromium hydroxide green
Questions?
Call 1-800-443-0602
Distributed by:
COLGATE-PALMOLIVE CO., Distr.
Guaynabo, P.R. 00968-1705
PRINCIPAL DISPLAY PANEL - 127 g Bar Carton
Eliminates 99% of Bacteria
Protex®
For the Good Health of your Skin
LIMPIEZA PROFUNDA
With seaweed extract Helps remove dirt and oil that can cause pimples
DERMATOLOGIST
TESTED
Bar Soap
NET WT 4.5 OZ (127 g)