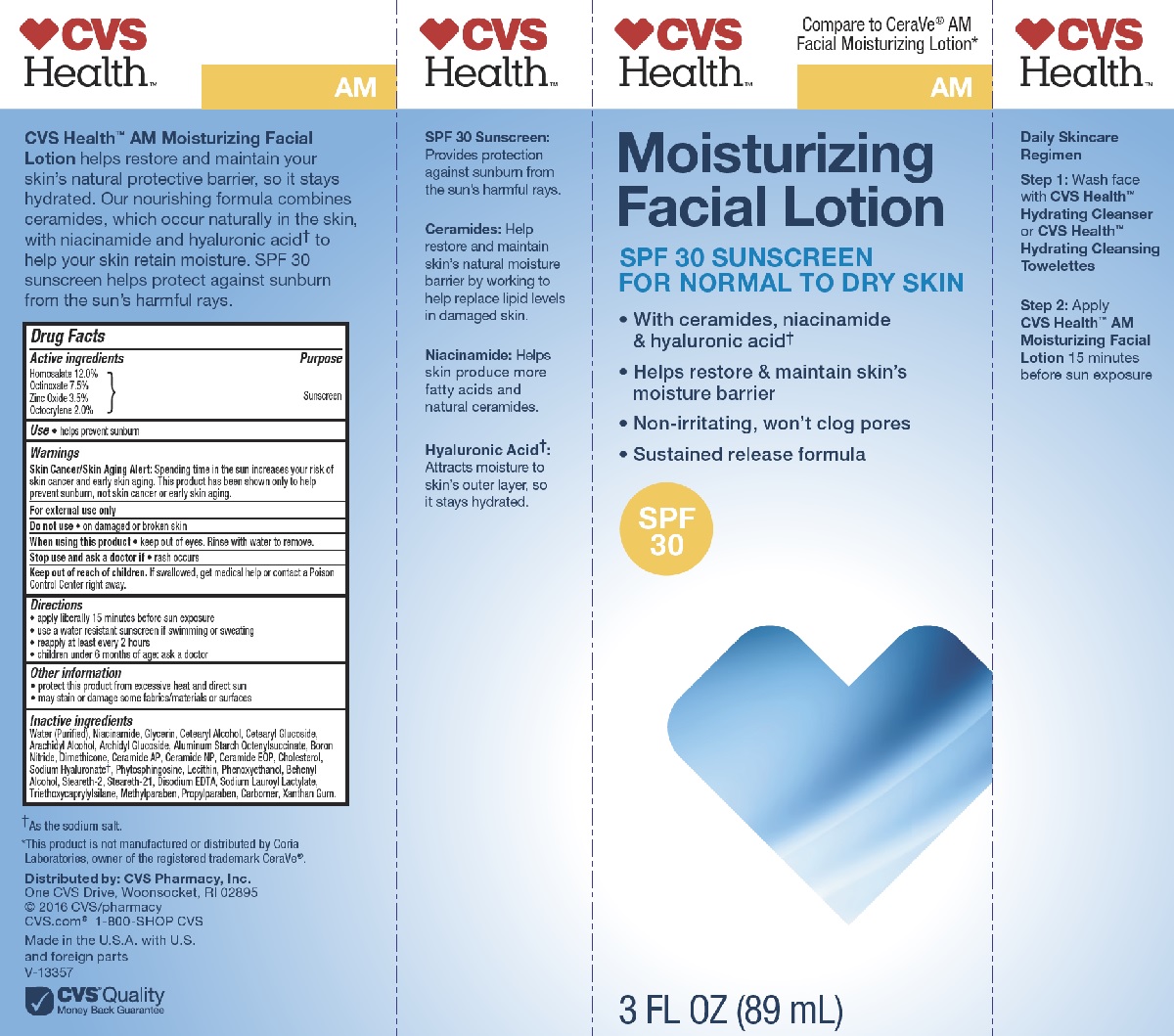
CVS HEALTH AM MOISTURIZING FACIAL- homosalate, octinoxate, zinc oxide, octocrylene lotion
CVS Health by
Drug Labeling and Warnings
CVS Health by is a Otc medication manufactured, distributed, or labeled by CVS Pharmacy. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredients
- Purpose
- Uses
- Warnings
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
-
Inactive ingredients
Water (Purified), Niacinamide, Glycerin, Cetearyl Alcohol, Cetearyl Glucoside, Arachidyl Alcohol, Archidyl Glucoside, Aluminum Starch Octenylsuccinate, Boron Nitride, Dimethicone, Ceramide AP, Ceramide NP, Ceramide EOP, Cholesterol, Sodium Hyaluronate, Phytosphingosine, Lecithin, Phenoxyethanol, Behenyl Alcohol, Steareth-2, Steareth-21, Disodium EDTA, Sodium Lauroyl Lactylate, Triethoxycaprylylsilane, Methylparaben, Propylparaben, Carbomer, Xanthan Gum.
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CVS HEALTH AM MOISTURIZING FACIAL
homosalate, octinoxate, zinc oxide, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69842-139 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 20 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 35 mg in 1 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 120 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ARACHIDYL GLUCOSIDE (UNII: 6JVW35JOOJ) BORON NITRIDE (UNII: 2U4T60A6YD) CHOLESTEROL (UNII: 97C5T2UQ7J) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) WATER (UNII: 059QF0KO0R) NIACINAMIDE (UNII: 25X51I8RD4) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) ARACHIDYL ALCOHOL (UNII: 1QR1QRA9BU) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARETH-2 (UNII: V56DFE46J5) METHYLPARABEN (UNII: A2I8C7HI9T) XANTHAN GUM (UNII: TTV12P4NEE) CERAMIDE AP (UNII: F1X8L2B00J) STEARETH-21 (UNII: 53J3F32P58) DIMETHICONE (UNII: 92RU3N3Y1O) DOCOSANOL (UNII: 9G1OE216XY) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) HYALURONATE SODIUM (UNII: YSE9PPT4TH) CERAMIDE NP (UNII: 4370DF050B) CERAMIDE 1 (UNII: 5THT33P7X7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69842-139-19 1 in 1 BOX 02/03/2017 1 NDC: 69842-139-18 89 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 02/03/2017 Labeler - CVS Pharmacy (062312574) Registrant - Fruit of the Earth, Inc. (079559467) Establishment Name Address ID/FEI Business Operations Fruit Of The Earth Research Laboratories, Inc. 008193513 manufacture(69842-139)
Trademark Results [CVS Health]
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.