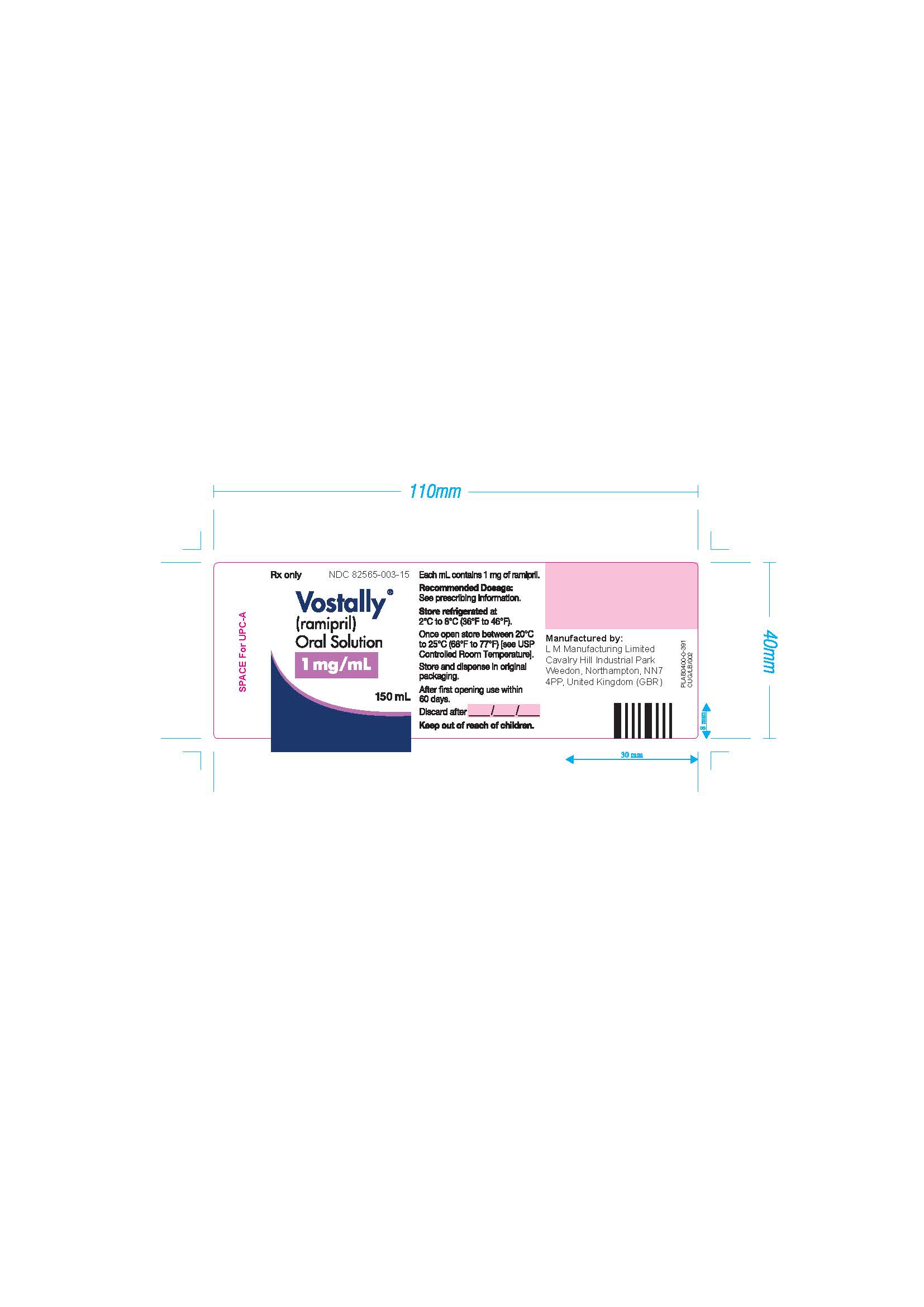
VOSTALLY by L M Manufacturing Limited Vostally
VOSTALLY by
Drug Labeling and Warnings
VOSTALLY by is a Prescription medication manufactured, distributed, or labeled by L M Manufacturing Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
VOSTALLY- ramipril oral solution liquid
L M Manufacturing Limited
----------
Vostally
| VOSTALLY
ramipril oral solution liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - L M Manufacturing Limited (220231510) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| L M Manufacturing Limited | 220231510 | analysis(82565-003) , manufacture(82565-003) | |
Revised: 1/2026
Document Id: 47de9205-fa5f-ff64-e063-6294a90ae710
Set id: 47de9205-fa5e-ff64-e063-6294a90ae710
Version: 1
Effective Time: 20260108
Trademark Results [VOSTALLY]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VOSTALLY 98200124 not registered Live/Pending |
Rosemont Pharmaceuticals Limited 2023-09-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.