ACTOGAIN- ractopamine hydrochloride powder
Actogain by
Drug Labeling and Warnings
Actogain by is a Animal medication manufactured, distributed, or labeled by Zoetis Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
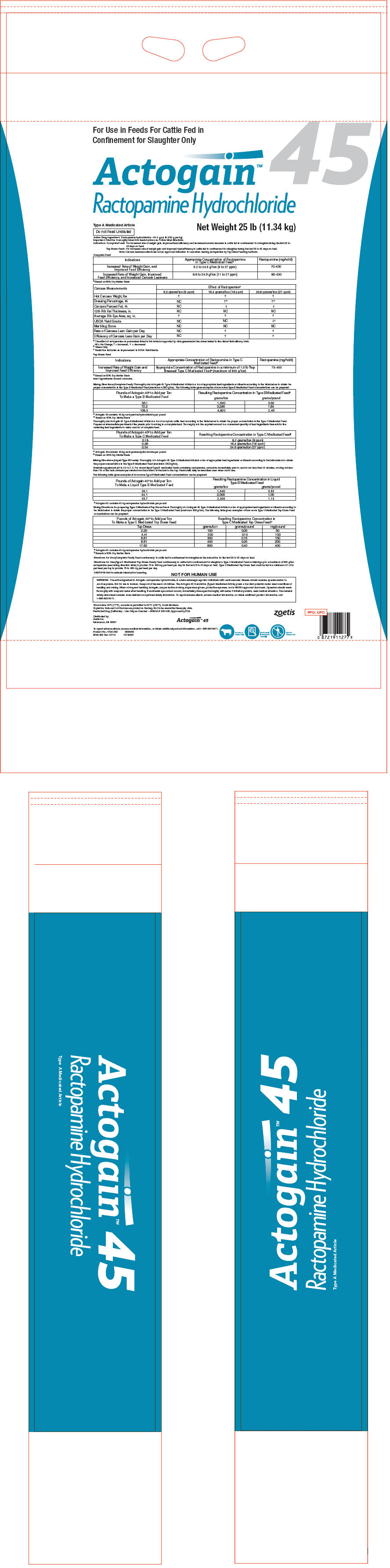
Active Drug Ingredient: Ractopamine hydrochloride – 45.4 g per lb (100 g per kg) Important: Must be thoroughly mixed into feeds before use. Follow label directions. Indication: Complete Feed: For increased rate of weight gain, improved feed efficiency and increased carcass leanness in cattle fed in confinement for slaughter during the last 28 to 42 days on feed. Top Dress Feed: For increased rate of weight gain and improved feed efficiency in cattle fed in confinement for slaughter during the last 28 to 42 days on feed. Note: Carcass leanness effects are not an approved indication for use when feeding ractopamine by Top Dress Feeding methods. -
Complete Feed
Indications Appropriate Concentration of Ractopamine in Type C Medicated Feed* Ractopamine (mg/hd/d) - * Based on 90% Dry Matter Basis
Increased Rate of Weight Gain, and Improved Feed Efficiency 8.2 to 24.6 g/ton (9 to 27 ppm) 70-430 Increased Rate of Weight Gain, Improved Feed Efficiency, and Increased Carcass Leanness 9.8 to 24.6 g/ton (11 to 27 ppm) 90-430 Carcass Measurements Effect of Ractopamine* 8.2 grams/ton (9 ppm) 16.4 grams/ton (18 ppm) 24.6 grams/ton (27 ppm) NC= No Change, ↑= increased, ↓ = decreased - * The effect of ractopamine on parameters listed in this table is supported by data generated at the doses tested in the clinical field efficacy trials.
- † Steers Only
- ‡ Reduction indicates an improvement in USDA Yield Grade.
Hot Carcass Weight, lbs ↑ ↑ ↑ Dressing Percentage, % NC ↑† ↑† Carcass Percent Fat, % NC ↓ ↓ 12th Rib Fat Thickness, in. NC NC NC Average Rib Eye Area, sq. in. ↑ ↑ ↑ USDA Yield Grade NC NC ↓‡ Marbling Score NC NC NC Rate of Carcass Lean Gain per Day NC ↑ ↑ Efficiency of Carcass Lean Gain per Day NC ↑ ↑ -
Top Dress Feed
Indications Appropriate Concentration of Ractopamine in Type C Medicated Feed* Ractopamine (mg/hd/d) - * Based on 90% Dry Matter Basis
Increased Rate of Weight Gain and Improved Feed Efficiency Appropriate Concentration of Ractopamine in a minimum of 1.0 lb Top Dressed Type C Medicated Feed* (maximum of 800 g/ton) 70-400 Inert Ingredients: Ground corncobs.
-
SPL UNCLASSIFIED SECTION
Mixing Directions (Complete Feed): Thoroughly mix Actogain 45 Type A Medicated Article in a ton of appropriate feed ingredients or diluents according to the table below to obtain the proper concentration in the Type B Medicated Feed (maximum 4,920 g/ton). The following table gives examples of how some Type B Medicated Feed concentrations can be prepared:
Pounds of Actogain 45* to Add per Ton To Make a Type B Medicated Feed Resulting Ractopamine Concentration in Type B Medicated Feed† grams/ton grams/pound - * Actogain 45 contains 45.4g ractopamine hydrochloride per pound
- † Based on 90% Dry Matter Basis
36.1 1,640 0.82 72.2 3,280 1.64 108.3 4,920 2.46 Thoroughly mix Actogain 45 Type A Medicated Article in a ton of complete cattle feed according to the table below to obtain the proper concentration in the Type C Medicated Feed. Prepare an intermediate pre-blend of the premix prior to mixing in a complete feed. Thoroughly mix the required amount in a convenient quantity of feed ingredients then add to the remaining feed ingredients to make one ton of complete feed.
Pounds of Actogain 45* to Add per Ton To Make a Type C Medicated Feed Resulting Ractopamine Concentration in Type C Medicated Feed† - * Actogain 45 contains 45.4g ractopamine hydrochloride per pound
- † Based on 90% Dry Matter Basis
0.18 8.2 grams/ton (9 ppm) 0.36 16.4 grams/ton (18 ppm) 0.54 24.6 grams/ton (27 ppm) -
SPL UNCLASSIFIED SECTION
Mixing Directions (Liquid Type B Feeds): Thoroughly mix Actogain 45 Type A Medicated Article in a ton of appropriate feed ingredients or diluents according to the table below to obtain the proper concentration in the Type B Medicated Feed (maximum 2300 g/ton).
Maintain supplement pH to 4.5 to 7.5. For stored liquid Type B medicated feeds containing ractopamine, recirculate immediately prior to use for not less than 10 minutes, moving not less than 1% of the tank contents per minute from the bottom of the tank to the top. Recirculate daily as described even when not in use.
The following table gives examples of how some Type B Medicated Feed concentrations can be prepared:
-
SPL UNCLASSIFIED SECTION
Mixing Directions for preparing Type C Medicated Top Dress Feed: Thoroughly mix Actogain 45 Type A Medicated Article in a ton of appropriate feed ingredients or diluents according to the table below to obtain the proper concentration in the Type C Medicated Feed (maximum 800 g/ton). The following table gives examples of how some Type C Medicated Top Dress Feed concentrations can be prepared:
Pounds of Actogain 45* to Add per Ton To Make a Type C Medicated Top Dress Feed Resulting Ractopamine Concentration in Type C Medicated Top Dress Feed† Top Dress grams/ton grams/pound mg/pound - * Actogain 45 contains 45.4g ractopamine hydrochloride per pound
- † Based on 90% Dry Matter Basis
2.20 100 0.05 50 4.41 200 0.10 100 6.61 300 0.15 150 8.81 400 0.20 200 17.62 800 0.40 400 -
DOSAGE & ADMINISTRATION
Directions for Use (Complete Feed): Feed continuously to cattle fed in confinement for slaughter as the sole ration for the last 28 to 42 days on feed.
Directions for Use (Type C Medicated Top Dress Feed): Feed continuously to cattle fed in confinement for slaughter a Type C Medicated Feed containing up to a maximum of 800 g/ton ractopamine (see mixing direction table) to provide 70 to 400 mg per head per day for the last 28 to 42 days on feed. Type C Medicated Top Dress feed must be fed in a minimum of 1.0 lb per head per day to provide 70 to 400 mg per head per day.
- PRECAUTIONS
-
BOXED WARNING
(What is this?)
WARNING: The active ingredient in Actogain, ractopamine hydrochloride, is a beta-adrenergic agonist. Individuals with cardiovascular disease should exercise special caution to avoid exposure. Not for use in humans. Keep out of the reach of children. The Actogain 45 formulation (Type A Medicated Article) poses a low dust potential under usual conditions of handling and mixing. When mixing and handling Actogain, use protective clothing, impervious gloves, protective eye wear, and a NIOSH-approved dust mask. Operators should wash thoroughly with soap and water after handling. If accidental eye contact occurs, immediately rinse eyes thoroughly with water. If irritation persists, seek medical attention. The material safety data sheet contains more detailed occupational safety information. To report adverse effects, access medical information, or obtain additional product information, call 1-888-963-8471.
- STORAGE AND HANDLING
- SPL UNCLASSIFIED SECTION
- ADVERSE REACTIONS
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 11.34 kg Bag
-
INGREDIENTS AND APPEARANCE
ACTOGAIN
ractopamine hydrochloride powderProduct Information Product Type OTC TYPE A MEDICATED ARTICLE ANIMAL DRUG Item Code (Source) NDC: 54771-8728 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RACTOPAMINE HYDROCHLORIDE (UNII: 309G9J93TP) (RACTOPAMINE - UNII:57370OZ3P1) RACTOPAMINE HYDROCHLORIDE 100 g in 1 kg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SOYBEAN OIL (UNII: 241ATL177A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54771-8728-1 11.34 kg in 1 BAG Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200548 07/18/2013 Labeler - Zoetis Inc. (828851555)
Trademark Results [Actogain]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ACTOGAIN 85576523 4837048 Live/Registered |
ZOETIS SERVICES LLC 2012-03-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.