BRINEURA- cerliponase alfa kit
Brineura by
Drug Labeling and Warnings
Brineura by is a Prescription medication manufactured, distributed, or labeled by BioMarin Pharmaceutical Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BRINEURA safely and effectively. See full prescribing information for BRINEURA.
BRINEURA (cerliponase alfa) injection, for intraventricular use
Initial U.S. Approval: 2017INDICATIONS AND USAGE
Brineura is a hydrolytic lysosomal N-terminal tripeptidyl peptidase indicated to slow the loss of ambulation in symptomatic pediatric patients 3 years of age and older with late infantile neuronal ceroid lipofuscinosis type 2 (CLN2), also known as tripeptidyl peptidase 1 (TPP1) deficiency. (1)
DOSAGE AND ADMINISTRATION
- Aseptic technique must be strictly observed during preparation and administration. (2.1)
- Brineura should be administered by, or under the direction of, a physician experienced in intraventricular administration. (2.1)
- Prior to each infusion, inspect the scalp for signs of intraventricular access device leakage, failure or potential infection.
- Obtain a sample of CSF for cell count and culture prior to each infusion and if clinically indicated. (2.1)
- Brineura is administered to the cerebrospinal fluid (CSF) by infusion via a surgically implanted reservoir and catheter. (2.1)
- Pre-treatment of patients with antihistamines with or without antipyretics or corticosteroids is recommended 30 to 60 minutes prior to the start of infusion. (2.2)
- The recommended dosage is 300 mg administered once every other week as an intraventricular infusion followed by infusion of Intraventricular Electrolytes over approximately 4.5 hours. (2.2)
- For complete information on preparation, specific intraventricular access device for use, and administration, see the full prescribing information. (2.1, 2.3, 2.4, 2.5)
DOSAGE FORMS AND STRENGTHS
Injection: Brineura 150 mg/5 mL (30 mg/mL) solution, two single‑dose vials per carton co-packaged with Intraventricular Electrolytes Injection 5 mL in a single-dose vial. (3)
CONTRAINDICATIONS
-
Any sign or symptom of acute or unresolved localized infection on or around the device insertion site (e.g. cellulitis or abscess); or suspected or confirmed CNS infection (e.g., cloudy CSF or positive CSF gram stain, or meningitis). (4)
-
Any acute intraventricular access device-related complication (e.g., leakage, extravasation of fluid, or device failure). (4)
-
Patients with ventriculoperitoneal shunts. (4)
WARNINGS AND PRECAUTIONS
-
Meningitis and Other Intraventricular Access Device-Related Infections: Monitor the device insertion site for signs of infection. (4, 5.1)
-
Intraventricular Access Device-Related Complications: Consult a neurosurgeon for any complications with the implanted device. In case of device-related complication, discontinue the infusion and refer to the device labeling for further instructions. (4, 5.2)
-
Cardiovascular Adverse Reactions: Monitor vital signs before, during, and post-infusion. Monitor Electrocardiogram (ECG) in patients with a history of bradycardia, conduction disorder, or with structural heart disease, during the infusion. In patients without cardiac abnormalities, perform regular 12-lead ECG evaluations every 6 months. (2.5, 5.3)
-
Hypersensitivity Reactions Including Anaphylaxis: Observe patients during and after the infusion. If a severe hypersensitivity reaction occurs, immediately stop the infusion and initiate appropriate treatment. (5.4)
ADVERSE REACTIONS
Most common adverse reactions (≥8%) are: pyrexia, ECG abnormalities, decreased CSF protein, vomiting, seizures, device-related complications,
hypersensitivity, increased CSF protein, hematoma, headache, irritability, pleocytosis, device-related infections, bradycardia, feeling jittery, and
hypotension. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact BioMarin at 1-866-906-6100 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
RECENT MAJOR CHANGES
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Preparation and Administration Information
2.2 Dosage
2.3 Method of Administration
2.4 Preparation for Infusion
2.5 Intraventricular Infusion Procedure
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Meningitis and Other Intraventricular Access Device-Related Infections
5.2 Intraventricular Access Device-Related Complications
5.3 Cardiovascular Adverse Reactions
5.4 Hypersensitivity Reactions Including Anaphylaxis
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
6.3 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Preparation and Administration Information
-
Aseptic technique must be strictly observed during preparation and administration.
-
Brineura should be administered by, or under the direction of, a physician experienced in intraventricular administration.
-
Prior to each infusion of Brineura, inspect the scalp for signs of intraventricular access device leakage, failure or potential infection [see Warnings and Precautions (5.1, 5.2)].
-
Prior to each infusion of Brineura and when clinically indicated, obtain a sample of CSF for cell count and culture [see Warnings and Precautions (5.1)].
-
Brineura is administered into the cerebrospinal fluid (CSF) by infusion via a surgically implanted reservoir and catheter (intraventricular access device). Brineura is intended to be administered via the Codman® HOLTER RICKHAM Reservoirs (Part Numbers: 82-1625, 82-1621, 82-1616) with the Codman® Ventricular Catheter (Part Number: 82-1650). The intraventricular access device must be implanted prior to the first infusion. It is recommended that the first dose be administered at least 5 to 7 days after device implantation.
-
Brineura is intended to be administered with the B Braun Perfusor® Space Infusion Pump System (Product Code: 8713030U). If an alternative pump must be used, the essential performance requirements for this syringe pump used to deliver Brineura are as follows:
-
Delivery rate of 2.5 mL/hr with delivery accuracy of +/- 1 mL/hr
-
Compatible with 20 mL syringes provided in the Administration Kit for use with Brineura
-
Occlusion alarm setting to ≤ 281 mm Hg
-
Cleared for intraventricular route of administration
-
-
Administer Brineura and the Intraventricular Electrolytes using the provided Administration Kit for use with Brineura components [see How Supplied/Storage and Handling (16)].
2.2 Dosage
The recommended dosage of Brineura in pediatric patients 3 years of age and older is 300 mg administered once every other week by intraventricular infusion. Administer Brineura first followed by infusion of the Intraventricular Electrolytes each at an infusion rate of 2.5 mL/hr. The complete Brineura infusion, including the required infusion of Intraventricular Electrolytes, is approximately 4.5 hours.
Pre-treatment of patients with antihistamines with or without antipyretics or corticosteroids is recommended 30 to 60 minutes prior to the start of infusion.
2.3 Method of Administration
Brineura and the Intraventricular Electrolytes must only be administered by the intraventricular route, using the provided Administration Kit for use with Brineura. Each vial of Brineura and Intraventricular Electrolytes is intended for a single dose only.
Each infusion consists of 10 mL of Brineura followed by 2 mL of Intraventricular Electrolytes. The complete infusion must be administered using an infusion set with a 0.2 micron inline filter. The Intraventricular Electrolytes are used to flush the infusion line, port needle, and intraventricular access device in order to fully administer Brineura and to maintain patency of the intraventricular access device.
2.4 Preparation for Infusion
Gather supplies:
-
Brineura and Intraventricular Electrolytes Injection vials (package 1 of 2) [see How Supplied/Storage and Handling (16)]
-
Administration Kit for use with Brineura (package 2 of 2) [see How Supplied/Storage and Handling (16)]
-
Syringe pump (not supplied)
Inspect the Administration Kit infusion components to ensure the components are in the individual packages and have not been compromised.
Thaw Brineura and Intraventricular Electrolytes Injection vials at room temperature for approximately 60 minutes. Do not thaw or warm vials any other way. Do not shake vials. Condensation will occur during thawing period. Do not re‑freeze vials or freeze syringes containing Brineura or Intraventricular Electrolytes.
Inspect fully thawed Brineura and Intraventricular Electrolytes Injection vials. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Brineura is a clear to slightly opalescent and colorless to pale yellow solution. Intraventricular Electrolytes is a clear to colorless solution. Do not use if the solutions are discolored or if there is other foreign particulate matter in the solutions. Brineura vials may occasionally contain thin translucent fibers or opaque particles. These naturally occurring particles are cerliponase alfa. These particles are removed via the 0.2 micron inline filter without having a detectable effect on the purity or strength of Brineura. Intraventricular Electrolytes may contain particles, which appear during the thaw period; however, these dissolve when the solution reaches room temperature.
2.5 Intraventricular Infusion Procedure
Intraventricular Infusion of Brineura
Figure 1 represents the intraventricular infusion system set up. Use aseptic technique during the infusion. Follow the steps below to proceed with the intraventricular infusion.
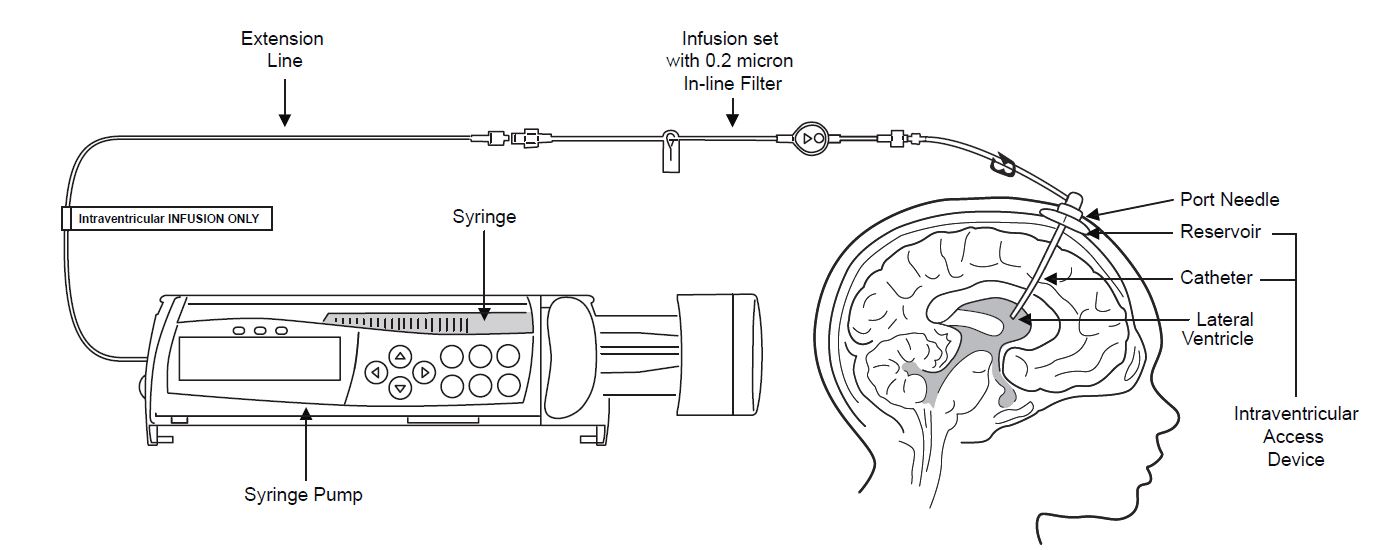
Figure 1
1. Use aseptic technique when preparing the Brineura syringe for infusion. Label one sterile syringe “Brineura” and attach the syringe needle. Remove the green flip-off caps from the two Brineura vials. Use the “Brineura” labeled syringe to withdraw a total of 10 mL from the Brineura vials. Do not dilute Brineura. Do not mix Brineura with any other drug.
2. Label the infusion line “intraventricular infusion only” (see Figure 1).
3. Attach the syringe containing Brineura to the extension line (see Figure 2). Then connect the extension line to the infusion set with a 0.2 micron inline filter (see Figure 1).
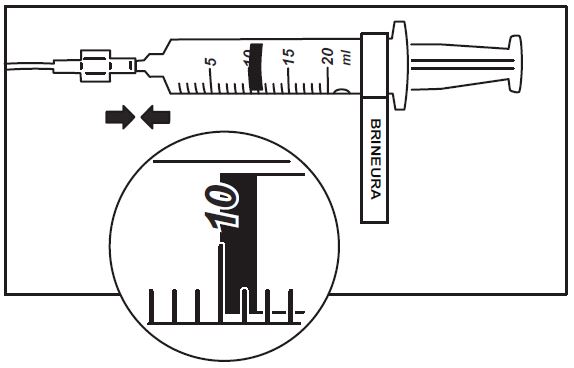
Figure 2
4. Prime the infusion components with Brineura.
5. Inspect scalp for signs of intraventricular access device leakage or failure and for potential infections [see Warnings and Precautions (5.1, 5.2)].
6. Prepare the scalp for intraventricular infusion per institution standard of care.
7. Insert port needle into intraventricular access device (see Figure 3).
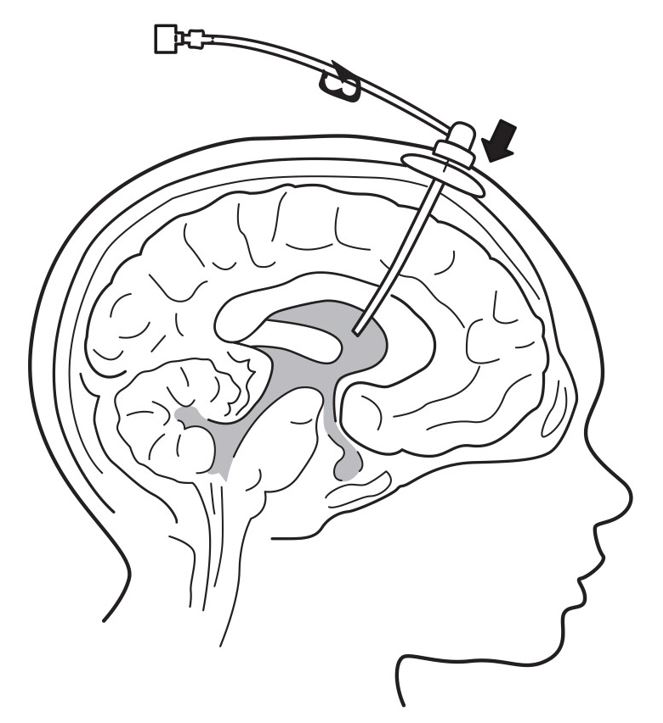
Figure 3
8. Connect a separate empty sterile single-use luer lock syringe, no larger than 3 mL (not provided) to the port needle. Withdraw 0.5 mL to 1 mL of CSF to check patency of intraventricular access device (see Figure 4) and send specimen for culture.
- Do not return CSF to intraventricular access device.
- Routinely send CSF samples for infection monitoring [see Warnings and Precautions (5.1)].
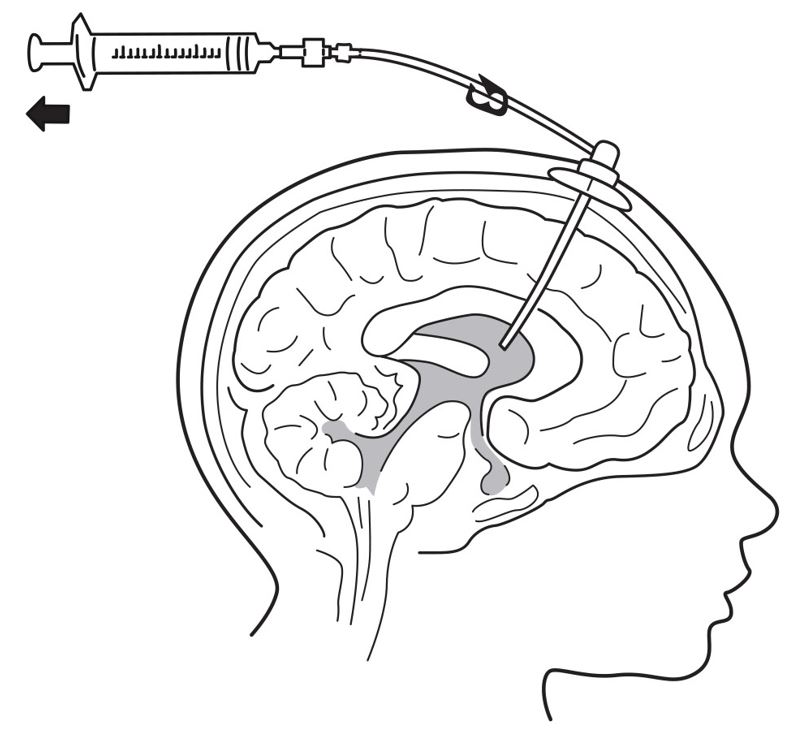
Figure 4
9. Attach the infusion set with 0.2 micron inline filter to the port needle (see Figure 1).
- Secure the components per institution standard of care.
10. Place the syringe containing Brineura into the syringe pump and program pump to deliver at an infusion rate of 2.5 mL per hour. Set the occlusion alarm setting to alert at pressure ≤ 281 mm Hg. See syringe pump operating manual for details. Do not deliver as a bolus or manually.
11. Administer pre-medication 30 to 60 minutes prior to the start of infusion [see Dosage and Administration (2.2)].
12. Monitor vital signs (blood pressure, heart rate) prior to the start of infusion, periodically during infusion, and post-infusion [see Warnings and Precautions (5.3)].
13. Initiate infusion of Brineura at a rate of 2.5 mL per hour.
14. Periodically inspect the infusion system during the infusion for signs of leakage or delivery failure [see Warnings and Precautions (5.2)].
15. When the Brineura infusion is complete, detach and remove the empty syringe from the pump and disconnect from the tubing (see Figure 5). Proceed to Step 16 for Intraventricular Electrolytes infusion.
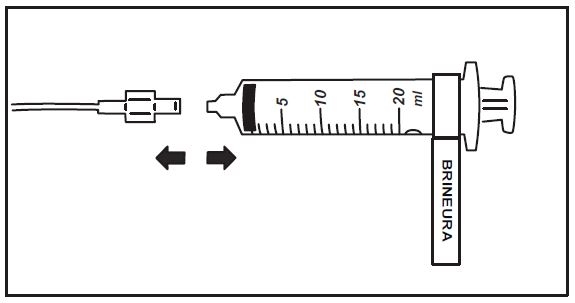
Figure 5
Intraventricular Infusion of Intraventricular Electrolytes
Administer the Intraventricular Electrolytes provided after Brineura infusion is complete.
16. Use aseptic technique when preparing the Intraventricular Electrolytes syringe for infusion. Label one sterile syringe “Intraventricular Electrolytes” and attach the syringe needle. Remove the yellow flip-off cap from the Intraventricular Electrolytes Injection vial. Withdraw 2 mL of Intraventricular Electrolytes. Discard the remaining unused portion.
17. Attach the syringe to the extension line (see Figure 6).
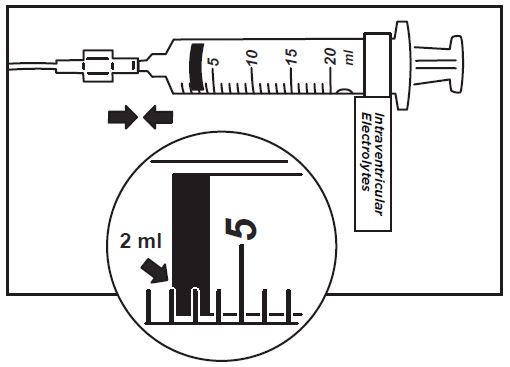
Figure 6
18. Place the syringe containing Intraventricular Electrolytes into the syringe pump and program pump to deliver at an infusion rate of 2.5 mL per hour. Set the occlusion alarm setting to alert at pressure ≤ 281 mm Hg. See syringe pump operating manual for details. Do not deliver as a bolus or manually.
19. Initiate infusion of Intraventricular Electrolytes at a rate of 2.5 mL per hour.
20. Periodically inspect the infusion system during the infusion for signs of leakage or delivery failure.
21. When the Intraventricular Electrolytes infusion is complete, detach and remove the empty syringe from the pump and disconnect from the infusion line.
22. Remove the port needle. Apply gentle pressure and bandage the infusion site per institution standard of care.
Dispose of the infusion components, needles, unused solutions and other waste materials in accordance with local requirements.
Storage of Thawed Product
Use thawed Brineura and Intraventricular Electrolytes immediately. If not used immediately, store unopened vials in the refrigerator at 2°C to 8°C and use within 24 hours.
Storage of Product in Syringes
Use product held in labeled syringes immediately. If not used immediately, store product held in labeled syringes in the refrigerator at 2°C to 8°C up to 4 hours prior to infusion.
-
-
3 DOSAGE FORMS AND STRENGTHS
Injection: Brineura 150 mg/5 mL (30 mg/mL) solution, two single-dose vials per carton co-packaged with Intraventricular Electrolytes Injection 5 mL in a single-dose vial. Brineura is a clear to slightly opalescent and colorless to pale yellow solution. Intraventricular Electrolytes is a clear to colorless solution [see How Supplied/Storage and Handling (16)].
-
4 CONTRAINDICATIONS
Brineura is contraindicated in patients with:
- any sign or symptom of acute, unresolved localized infection on or around the device insertion site (e.g. cellulitis or abscess); or suspected or confirmed CNS infection (e.g. cloudy CSF or positive CSF gram stain, or meningitis) [see Warnings and Precautions (5.1)].
- any acute intraventricular access device-related complication (e.g., leakage, extravasation of fluid, or device failure) [see Warnings and Precautions (5.2)].
- ventriculoperitoneal shunts.
-
5 WARNINGS AND PRECAUTIONS
5.1 Meningitis and Other Intraventricular Access Device-Related Infections
Bacterial meningitis requiring antibiotic treatment and removal of the device was reported during postmarketing use of Brineura. Additionally, in clinical trials and during postmarketing use there were reports of other device-related clinical infections which were confirmed by positive CSF cultures, treated with antibiotics and removal of the device, and in which patients resumed treatment with Brineura after the device was replaced [see Adverse Reactions (6.1)]. In all cases, antibiotics were administered, the intraventricular access devices were removed and replaced, and patients resumed treatment with Brineura.
The signs and symptoms of infections may not be readily apparent in patients with CLN2 disease. To reduce the risk of infectious complications, Brineura should be administered by, or under the direction of, a physician experienced in intraventricular administration [see Dosage and Administration (2.3)]. Prior to each infusion of Brineura, and when clinically indicated, obtain a sample of CSF for cell count and culture. Do not administer Brineura if there are localized signs of infection on or around the device insertion site, such as erythema, tenderness, or discharge or suspected or confirmed CNS infection (e.g. cloudy CSF or positive CSF gram stain, or meningitis) [see Contraindications (4)].
Healthcare providers should be vigilant for the development of signs and symptoms of infection, including meningitis, during treatment with Brineura and monitor the device insertion site for signs of infection.
5.2 Intraventricular Access Device-Related Complications
During the clinical trial and in postmarketing reports, intraventricular access device-related complications were reported (e.g., device leakage, device failure extravasation of CSF fluid, or bulging of the scalp around or above the intraventricular access device) [see Adverse Reactions (6.2)]. For any complications with the implanted intraventricular access device, consult with a neurosurgeon to confirm the integrity or performance of the device. In case of intraventricular access device-related complications, discontinue the Brineura infusion and refer to the device manufacturer’s labeling for further instructions [see Contraindications (4)].
Material degradation of the intraventricular access device reservoir was reported after approximately 4 years of administration, which may impact the effective and safe use of the device. During benchtop testing such material degradation was recognized after approximately 105 perforations of the intraventricular access device. The intraventricular access device should be replaced prior to 4 years of single-puncture administrations, which equates to approximately 105 administrations of Brineura.
5.3 Cardiovascular Adverse Reactions
Monitor vital signs (blood pressure, heart rate) before infusion starts, periodically during infusion, and post-infusion in a healthcare setting [see Dosage and Administration (2.5)]. Upon completion of the infusion, clinically assess the patient status. Continued observation may be necessary if clinically indicated.
Perform electrocardiogram (ECG) monitoring during infusion in patients with a history of bradycardia, conduction disorder, or with structural heart disease, as some patients with CLN2 disease may develop conduction disorders or heart disease. In patients without cardiac abnormalities, regular 12-lead ECG evaluations should be performed every 6 months.
In the clinical studies, hypotension was reported in 2 (8%) patients, which occurred during or up to eight hours after Brineura infusion. Patients did not require alteration in treatment, and reactions resolved spontaneously or after intravenous fluid administration [see Adverse Reactions (6.1)].
5.4 Hypersensitivity Reactions Including Anaphylaxis
Hypersensitivity reactions including anaphylaxis have been reported in Brineura-treated patients during clinical studies and postmarketing use [see Adverse Reactions (6.1, 6.3)]. In clinical trials, a total of 11 (46%) patients experienced hypersensitivity reactions during the infusion or within 24 hours of completion of the infusion. Patients in clinical trials were routinely pre-medicated with antihistamines with or without antipyretics or corticosteroids, prior to infusion of Brineura. During postmarketing use, anaphylactic reactions occurred during or within several hours of Brineura infusion. Epinephrine was administered in one case. Patients received subsequent Brineura infusions without recurrence of anaphylaxis.
Due to the potential for anaphylaxis, appropriate medical support should be readily available when Brineura is administered. Observe patients closely during and after the infusion. If a severe hypersensitivity reaction or anaphylaxis occurs, immediately discontinue the infusion and initiate appropriate medical treatment. Inform patients/caregivers of the signs and symptoms of hypersensitivity reactions and anaphylaxis and instruct them to seek immediate medical care should signs and symptoms occur.
The management of hypersensitivity reactions should be based on the severity of the reaction and may include temporarily interrupting the infusion, and/or treatment with antihistamines, antipyretics, and/or corticosteroids. Consider the risks and benefits of readministration of Brineura following an anaphylactic reaction. If the decision is made to readminister Brineura after the occurrence of anaphylaxis, ensure appropriately trained personnel and equipment for emergency resuscitation (including epinephrine and other emergency medicines) are readily available during infusion. Initiate subsequent infusion at approximately one-half the initial infusion rate at which the anaphylactic reaction occurred.
-
6 ADVERSE REACTIONS
The following adverse reactions are described below and elsewhere in the labeling:
-
Meningitis and Other Intraventricular Access Device-Related Infections [see Warnings and Precautions (5.1)]
-
Intraventricular Access Device-Related Complications [see Warnings and Precautions (5.2)]
-
Cardiovascular Adverse Reactions [see Warnings and Precautions (5.3)]
-
Hypersensitivity Reactions Including Anaphylaxis [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
The safety of Brineura was evaluated in 24 patients with CLN2 disease who received at least one dose of Brineura in a clinical study with extension of up to 161 weeks [see Clinical Studies (14)]. Table 1 summarizes the most common adverse reactions that occurred in Brineura-treated patients through 96 weeks.
Table 1: Adverse Reactions Reported in ≥ 8% of Symptomatic Pediatric Patients with CLN2 Disease in the Brineura Single-Arm Clinical Study with Extension at Week 96
Adverse Reaction Patients Treated with Brineura
n=24 (%)
Pyrexia1 17 (71) ECG abnormalities2 17 (71) Decreased CSF protein 17 (71) Vomiting 15 (63) Seizures3 12 (50) Device-related complications4 12 (50) Hypersensitvity5 11 (46) Increased CSF protein 5 (21) Hematoma 5 (21) Headache 4 (17) Irritability 4 (17) Pleocytosis 4 (17) Device-related infection6 2 (8) Bradycardia 2 (8) Feeling jittery 2 (8) Hypotension 2 (8) 1Pyrexia includes: pyrexia and increased body temperature
2ECG abnormalities include: non-specific repolarization abnormality, notched QRS, ST segment elevation, biphasic T wave abnormality, supraventricular extrasystoles, bradycardia, sinus tachycardia, and intraventricular conduction delay
3Seizures include: atonic, generalized tonic-clonic, focal, and absence
4Device-related complications include device-related infection, delivery system-related complications (needle issues, device leakage, device malfunction, device difficult to use, etc.) and pleocytosis.
5Hypersensitivity includes: immune reactions and signs and symptoms observed concomitantly with hypersensitivity reactions including pyrexia, vomiting, pleocytosis or irritability [see Warnings and Precautions (5.4)]
6Device-related infections include: Propionibacterium acnes and Staphylococcus epidermidis
Description of Selected Adverse Reactions
Seizures
Seizures were reported in 12 of 24 (50%) patients. The seizure types reported include atonic, generalized tonic-clonic, focal, and absence. Seizures were managed with standard anti-convulsive therapies and did not result in discontinuation of Brineura treatment.
Device-Related Complications
Adverse reactions related to the device were observed in 12 of 24 (50%) of patients. Device-related adverse reactions include infection, delivery system-related complications, and pleocytosis. Nine out of 24 patients (38%) experienced adverse reactions, which involved complications of the non-implanted delivery system components. Four out of 24 patients (16%) had device-related adverse reactions, which required medical intervention, including two patients (8%) with intraventricular access device-related CNS infections, and one patient (4%) each with leakage of the intraventricular access device and pleocytosis. Device-related infections were diagnosed by increased CSF pleocytosis and microbiology culture and organism identification, without accompanying signs and symptoms of meningitis. Intraventricular access devices were replaced and infections were treated with antibiotics. Device-related complications did not result in discontinuation of Brineura treatment [see Warnings and Precautions (5.1, 5.2)].
Hematoma
Hematoma adverse reactions were reported in 5 (21%) patients treated with Brineura and presented as hematoma, post procedural hematoma, traumatic hematoma and subdural hematoma. Hematomas did not require treatment and did not interfere with Brineura infusion.
Hypersensitivity
Hypersensitivity reactions were reported in 11 out of 24 patients (46%) treated with Brineura during or within 24 hours after completion of the Brineura infusion, despite pre-medication with antihistamines with or without antipyretics or corticosteroids [see Warnings and Precautions (5.1)]. The most common manifestations observed concomitantly with hypersensitivity included pyrexia with vomiting, pleocytosis, or irritability, which are not consistent with classic immune mediated hypersensitivity. Symptoms resolved over time or with administration of antipyretics, antihistamines and/or corticosteroids and no patient discontinued treatment with Brineura.
One patient experienced hypoxia (decreased oxygen saturation less than 88% by pulse oximeter), 8 hours after Brineura infusion, followed by a low mean arterial pressure at 15 hours post infusion. Symptoms resolved after oxygen administration, airway repositioning and normal saline infusion. One patient reported decreased oxygen saturation (90% by pulse oximeter), 45 minutes after starting Brineura with associated low diastolic blood pressures. Hypoxia resolved after oxygen administration. No treatment was administered for the low diastolic blood pressure, which returned to normal while the patient continued to receive Brineura infusion without change to the infusion rate or dose.
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to cerliponase alfa in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
Anti-drug antibodies (ADAs) to cerliponase alfa were detected in both serum and CSF in 79% and 33%, respectively, of patients treated with Brineura for up to 161 weeks. Patients who experienced hypersensitivity adverse reactions were tested for drug-specific IgE and found to be negative, including three patients for whom grade 3 (severe) hypersensitivity adverse reactions were reported. No association was found between serum or CSF ADA titers and incidence or severity of hypersensitivity. Drug-specific neutralizing antibodies (NAb) have not been evaluated.
6.3 Postmarketing Experience
The following adverse reactions have been identified during post approval use of Brineura. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Infections and infestations: Bacterial meningitis [see Warnings and Precautions (5.1)].
Immune system disorders: Anaphylactic reaction characterized by acute pyrexia, respiratory distress (bronchospasm, hypoxemia, perioral cyanosis), tachycardia, hypotension, diarrhea, and rash [see Warnings and Precautions (5.4)].
-
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on Brineura use in pregnant women to inform a drug-associated risk of pregnancy-related outcomes. Animal reproduction studies have not been conducted using cerliponase alfa.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of cerliponase alfa in human milk, the effects on the breastfed child, or the effects on milk production. The lack of clinical data during lactation precludes a clear determination of the risk of Brineura to an infant during lactation; therefore, the development and health benefits of breastfeeding should be considered along with the mother’s clinical need for Brineura and any potential adverse effects on the breastfed infant from Brineura or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness of Brineura have been established in pediatric patients 3 years of age and older. Pediatric use of Brineura to slow the loss of ambulation in symptomatic pediatric patients 3 years of age and older with late infantile neuronal ceroid lipofuscinosis type 2 (CLN2), also known as tripeptidyl peptidase 1 (TPP1) deficiency, is supported by a non-randomized single-arm dose escalation clinical study with extension in patients with CLN2 disease and compared to untreated patients with CLN2 disease from an independent natural history cohort [see Clinical Studies (14)]. Safety and effectiveness in patients less than 3 years of age have not been established.
-
11 DESCRIPTION
Cerliponase alfa is a purified human enzyme produced by recombinant DNA technology in a Chinese hamster ovary cell line. The active substance is a recombinant human tripeptidyl peptidase-1 (rhTPP1), a lysosomal exopeptidase. The primary activity of the mature enzyme is the cleavage of N-terminal tripeptides from a broad range of protein substrates.
Cerliponase alfa contains 544 amino acids with an average molecular mass of 59 kDa. The mature enzyme is 368 amino acids in length. There are 5 consensus N-glycosylation sites on rhTPP1 that contain high mannose, phosphorylated high mannose and complex glycosylation structures.
Brineura (cerliponase alfa) Injection and Intraventricular Electrolytes Injection are administered by intraventricular infusion. The solutions are sterile, nonpyrogenic, and free of foreign particulates. Brineura is a clear to slightly opalescent and colorless to pale yellow solution. Intraventricular Electrolytes is a clear to colorless solution.
Brineura and Intraventricular Electrolytes Injection are packaged in 10 mL clear Type 1 single-dose glass vials [see How Supplied/Storage and Handling (16)]. Each vial of Brineura provides 5 mL of solution containing 150 mg cerliponase alfa. Each vial of Intraventricular Electrolytes Injection provides 5 mL of solution. Both Brineura and Intraventricular Electrolytes Injection are formulated with the following excipients: calcium chloride dihydrate (1.05 mg); magnesium chloride hexahydrate (0.8 mg); potassium chloride (1.1 mg); sodium chloride (43.85 mg); sodium phosphate, dibasic, heptahydrate (0.55 mg); sodium phosphate, monobasic, monohydrate (0.4 mg); and Water for Injection, USP. The pH of the solution is between 6.2 to 6.8 for Brineura, and between 6.0 to 7.0 for Intraventricular Electrolytes Injection.
Each vial contains: sodium: 0.76 mEq, and potassium: 0.015 mEq.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
CLN2 disease is a neurodegenerative disease caused by deficiency of the lysosomal enzyme tripeptidyl peptidase-1 (TPP1), which catabolizes polypeptides in the CNS. TPP1 has no known substrate specificity. Deficiency in TPP1 activity results in the accumulation of lysosomal storage materials normally metabolized by this enzyme in the central nervous system (CNS), leading to progressive decline in motor function.
Cerliponase alfa (rhTTP1), a proenzyme, is taken up by target cells in the CNS and is translocated to the lysosomes through the Cation Independent Mannose-6-Phosphate Receptor (CI-MPR, also known as M6P/IGF2 receptor). Cerliponase alfa is activated in the lysosome and the activated proteolytic form of rhTPP1 cleaves tripeptides from the N-terminus of proteins.
12.3 Pharmacokinetics
The pharmacokinetics of cerliponase alfa were evaluated in patients with CLN2 disease who received intraventricular infusions of 30 mg (0.1 times the approved recommended dosage), 100 mg (approximately 0.3 times the approved recommended dosage), and 300 mg over approximately 4.5 hours once every other week.
Cerliponase alfa CSF exposure following the initial single dose administration of Brineura increased less than proportionally across doses of 30 mg, 100 mg, and 300 mg. There was no apparent accumulation of cerliponase alfa in CSF or plasma when Brineura was administered at a dose of 300 mg once every other week.
Cerliponase alfa pharmacokinetics have high inter-subject and intra-subject variability. Following intraventricular infusions of 300 mg of Brineura at Day 1, Week 5, and Week 13, the pharmacokinetic parameters in CSF and plasma were assessed in 14 patients and are summarized in Table 2.
Table 2: Pharmacokinetic Parameters of Cerliponase Alfa Following Intraventricular Infusion (approximately 4.5 hours in duration) of Brineura 300 mg Every Two Weeks
CSF
Parameter
Median [Min, Max]
Day 1
Week 5
Week 13
N
13
14
13
Tmax1, hr
4.5 [4.3, 5.8]
4.3 [3.8, 4.5]
4.3 [4.0, 4.5]
Cmax, mcg/mL
1260 [359, 4380]
1630 [376, 4670]
1390 [1110, 2340]
AUC0-t, mcg‑hr/mL
9290 [3660, 19000]
12400 [4620, 26200]
10500 [7000, 18200]
Vss, mL
245 [78.4, 909]
196 [85.4, 665]
186 [131, 257]
CL, mL/hr
32.3 [15.8, 81.9]
24.2 [11.4, 64.9]
28.7 [16.5, 42.9]
t1/2, hr
6.2 [5.5, 16.3]
7.4 [3.3, 9.5]
7.7 [5.1, 9.4]
Plasma2
N
12
12
9
Tmax1, hr
12.0 [4.3, 24.5]
12.0 [7.5, 24.2]
12.3 [4.3, 75.9]
Cmax, mcg/mL
1.3 [0.2, 3.9]
1.9 [0.2, 4.3]
1.0 [0.03, 2.6]
AUC0-t, mcg‑hr/mL
16.2 [1.1, 69.9]
40.1 [11.1, 78.9]
9.5 [0.2, 51.6]
CSF/Plasma
Ratio
N
11
12
9
Cmax
1200 [305, 4530]
809 [202, 9370]
1320 [541, 51200]
AUC0-t
393 [115, 1910]
340 [126, 1780]
1330 [167, 38900]
1Tmax expressed as time since start of ~4.5 hour infusion.
2Vss, CL, and t1/2 were not estimated due to insufficient plasma pharmacokinetic data
The estimated CSF volume of distribution of cerliponase alfa following intraventricular infusion of 300 mg of Brineura (median Vss = 245 mL) exceeds the typical CSF volume (100 mL).
Cerliponase alfa is a protein and is expected to be degraded through peptide hydrolysis.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
The efficacy of Brineura was assessed over 96 weeks in a non-randomized single-arm dose escalation clinical study with extension in symptomatic pediatric patients with late infantile neuronal ceroid lipofuscinosis type 2 (CLN2) disease, confirmed by TPP1 deficiency. Brineura-treated patients were compared to untreated patients from a natural history cohort. The Motor domain of a CLN2 Clinical Rating Scale was used to assess disease progression. Scores ranged from 3 (grossly normal) to 0 (profoundly impaired) with unit decrements representing milestone events in the loss of motor function (ability to walk or crawl). Due to the inability to establish comparability for the CLN2 Language domain ratings between the clinical study with extension and the natural history cohort, efficacy of Brineura for the Language domain cannot be established.
Twenty-four patients, aged 3 to 8 years were enrolled in the Brineura single-arm clinical study. Sixty-three percent of patients were female and 37% were male. Ninety-six percent of patients were Caucasian and 4% were Asian. One patient withdrew after week 1 due to inability to continue with study procedures; 23 patients were treated with Brineura 300 mg every other week for 48 weeks, and continued treatment during the extension period.
In the clinical study with extension, patients were assessed for decline in the Motor domain of the CLN2 Clinical Rating Scale at 48, 72 and 96 weeks. Decline was defined as having an unreversed (sustained) 2-category decline or an unreversed score of 0 in the Motor domain of the CLN2 Clinical Rating Scale. Patients’ responses to Brineura treatment were evaluated if at screening a combined Motor plus Language CLN2 score of less than 6 was recorded. Two patients with a combined Motor plus Language CLN2 score of 6 were excluded from the analyses; they maintained that score throughout the study period. The patient who terminated early was analyzed as having a decline at the time of termination. Data used in the analyses from the natural history cohort began at 36 months of age or greater and at the first time a Motor plus Language CLN2 score less than 6 was recorded.
Motor scores of the 22 Brineura-treated patients in the clinical study with extension were compared to scores of the independent natural history cohort that included 42 untreated patients who satisfied inclusion criteria for the clinical study. The results of logistic modeling with covariates (screening age, screening motor score, genotype: 0 key mutations (yes/no)), demonstrated the odds of Brineura-treated patients not having a decline by 96 weeks were 13 times the odds of natural history cohort patients not having a decline (Odds Ratio (95% CI): 13.1 (1.2, 146.9)).
Descriptive non-randomized comparison
In an unadjusted non-randomized comparison, of the 22 patients treated with Brineura and evaluated for efficacy at week 96, 21 (95%) did not decline, and only the patient who terminated early was deemed to have a decline in the Motor domain of the CLN2 Clinical Rating Scale. Results from the natural history cohort demonstrated progressive decline in motor function; of the 42 patients in the natural history cohort, 21 (50%) experienced an unreversed (sustained) 2-category decline or unreversed score of 0 in the Motor domain of the CLN2 Clinical Rating Scale over 96 weeks.
Given the non-randomized study design, a Cox Proportional Hazards Model adjusted for age, initial motor score, and genotype was used to evaluate time to unreversed 2-category decline or unreversed score of 0 in the Motor domain. This model showed a lesser decrease in motor function in the Brineura-treated patients when compared to the natural history cohort (see Figure 7).
Figure 7. Estimated Time to Unreversed (Sustained) 2-Category Decline or Unreversed Score of Zero in Motor Domain for Symptomatic Pediatric Patients in the Brineura Single-Arm Clinical Study with Extension and for Patients in a Natural History Cohort (Based on the Cox Proportional Hazards Model Adjusting for Covariates)
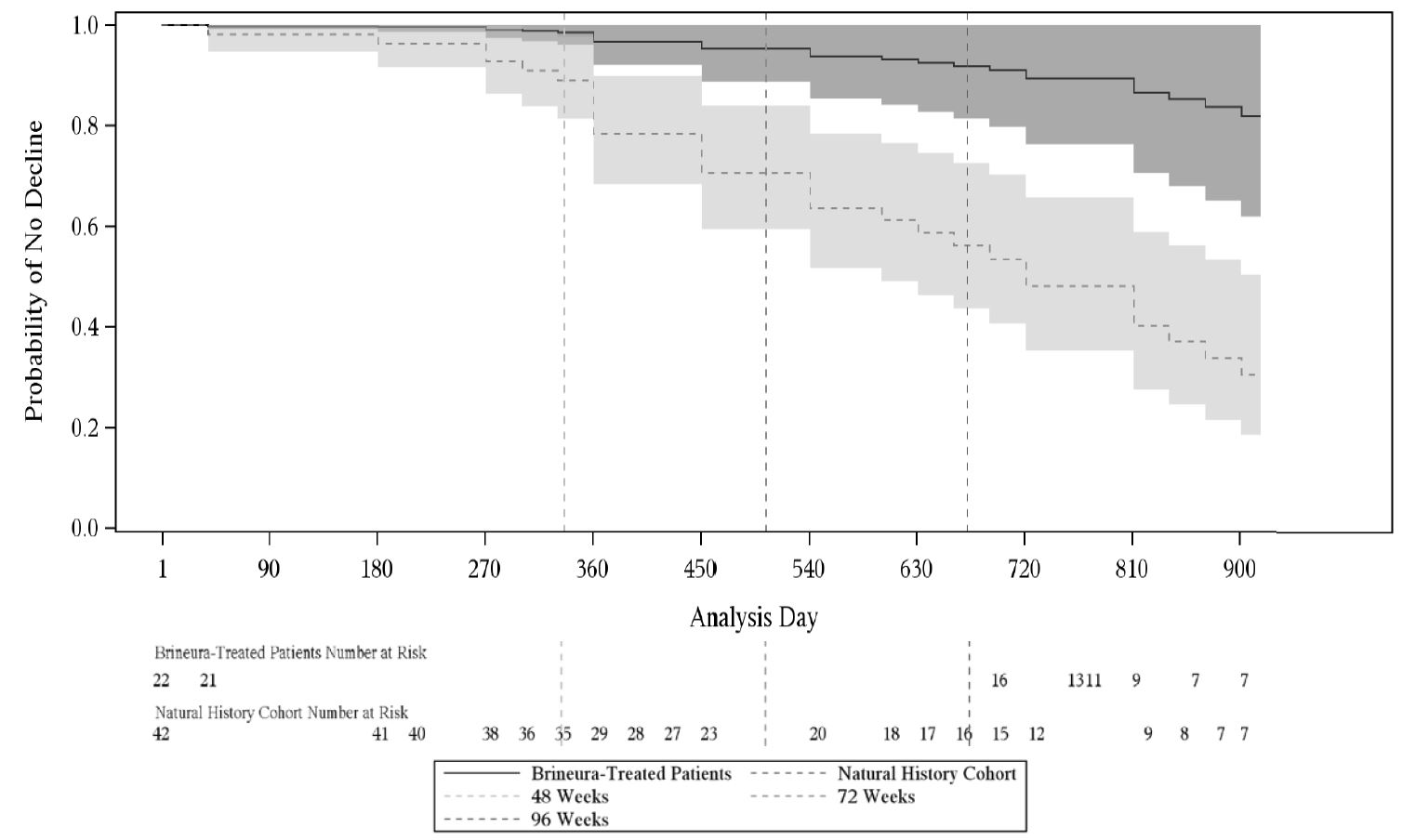
Shading represents 95% confidence intervals.
Follow-up for the natural history cohort begins at 36 months of age or greater and at the first time a Motor plus Language CLN2 score less than 6 was recorded.
The Brineura-treated population is the full population (N=24) minus two patients with baseline Motor plus Language CLN2 score = 6.
Covariates: screening age, screening Motor score, genotype: 0 key mutations (yes/no). “Screening age” was defined in the natural history cohort as the age at the first time a Motor plus Language CLN2 score less than 6 was recorded, and no earlier than 36 months of age. The “screening Motor score” of the natural history cohort was defined as the Motor score at the screening age.
Decline is defined as an unreversed (sustained) 2-category decline or unreversed score of 0 in the Motor domain of the CLN2 Clinical Rating Scale.
Motor Domain Scores: Matched Patients Only
To further assess efficacy, the 22 patients from the Brineura clinical study with a baseline combined Motor plus Language CLN2 score less than 6 were matched to 42 patients in the natural history cohort. Patients were matched based on the following covariates: baseline age at time of screening within 3 months, genotype (0, 1, or 2 key mutations), and baseline Motor domain CLN2 score at time of screening.
Using the Motor domain of the CLN2 Clinical Rating Scale, decline was defined as having an unreversed 2-category decline or an unreversed score of 0. At 96 weeks, the matched analysis based on 17 pairs demonstrated fewer declines in the Motor domain for Brineura-treated patients compared to untreated patients in the natural history cohort (see Table 3).
Table 3: Proportion of Matched Symptomatic Pediatric Patients with CLN2 Disease without Decline1 in the Brineura Single-Arm Clinical Study with Extension and in the Natural History Cohort assessed at Weeks 48, 72, and 96
Time Point/Period
Natural History Cohort
(N=17)Brineura-Treated
(N=17)Difference
Odds Ratio3
n (%)
n (%)
% (95% CI2)
OR (95% CI)
Follow-up through Week 48
13 (76)
16 (94)
18% (-19, 51)
4 (0.4, 200)
Follow-up through Week 72
11 (65)
16 (94)
29% (-7, 61)
5.9 (0.7, 250)
Follow-up through Week 96
6 (35)
16 (94)
59% (24, 83)
11 (1.6, 500)
1Decline is defined as an unreversed (sustained) 2-category decline or unreversed score of 0 in the Motor domain of the CLN2 Clinical Rating Scale.
2 Exact confidence interval for risk difference (Santner and Snell)
3Based on McNemar’s Exact test
Matched on baseline age at time of screening within 3 months, genotype (0, 1, or 2 key mutations), and baseline Motor domain CLN2 score at time of screening.
The Brineura-treated population is based on the full population minus two patients with baseline Motor plus Language CLN2 score = 6.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Brineura is supplied as a sterile, clear to slightly opalescent and colorless to pale yellow solution for intraventricular infusion and Intraventricular Electrolytes Injection is supplied as a clear to colorless solution for intraventricular infusion; both are included in package 1 of 2. The Administration Kit for use with Brineura is supplied separately as package 2 of 2 [see Dosage and Administration (2.2)].
Package 1 of 2
Each Brineura (cerliponase alfa) Injection vial has a green flip‑off cap (plastic), and contains 150 mg cerliponase alfa per 5 mL (30 mg/mL).
Each Intraventricular Electrolytes Injection vial has a yellow flip‑off cap (plastic), and contains 5 mL of solution.
Contents of Package 1
NDC Number
Brineura (cerliponase alfa) Injection (2 vials of 150 mg/5 mL)
Intraventricular Electrolytes Injection (1 vial, 5 mL)
68135-811-02
Package 2 of 2
The Administration Kit for use with Brineura is supplied separately and contains the following single-use, sterile infusion components:
- Two 20-mL syringes
- Two syringe needles (21 G, 25.4 mm)
- One extension line
- One infusion set with 0.2 micron inline filter
- One port needle (22 G, 16 mm)
Storage
Brineura (cerliponase alfa) Injection and Intraventricular Electrolytes Injection:
Store upright in a freezer (‑25°C to ‑15°C) in original carton to protect from light.
Administration Kit for use with Brineura:
Store in original carton separately from Brineura. Do not freeze.
-
17 PATIENT COUNSELING INFORMATION
- Intraventricular Access Device‑Related Infections
Advise patients and caregivers of the risk of device-related infections, including meningitis. Familiarize them with the signs and symptoms of these infections and instruct them to immediately contact their healthcare provider if an infection is suspected [see Warnings and Precautions (5.1)].
- Cardiovascular Adverse Reactions
Advise patients and caregivers that hypotension and/or bradycardia may occur during and following the infusion of Brineura. Instruct patients immediately to contact their healthcare provider if these reactions occur [see Warnings and Precautions (5.3)].
- Hypersensitivity Reactions Including Anaphylaxis
Advise patients and caregivers that hypersensitivity reactions related to Brineura treatment, including anaphylaxis characterized by fever, respiratory distress and rash may occur. Inform patients and caregivers of the signs and symptoms of anaphylaxis and instruct them to seek immediate medical care should signs and symptoms occur [see Warnings and Precautions (5.4)].
Manufactured by:
BioMarin Pharmaceutical Inc.
Novato, CA 94949
US License Number 1649
1-866-906-6100 (phone)Codman® and the B Braun Perfusor® are the registered trademarks of their respective owners and are not trademarks of BioMarin® Pharmaceutical Inc.
-
Brineura Carton
NDC: 68135-811-02
Brineura®
(cerliponase alfa)
Injection
150 mg/5 mL (30 mg/mL)
For Intraventricular Infusion only
Single-Dose Only
Each vial of Brineura® contains 150 mg cerliponase alfa in 5 mL of solution (30 mg/mL)
No preservatives
Discard unused portion.
Brineura® and Intraventricular Electrolytes must be administered with the Administration Kit provided.
Each carton contains:
2 vials, each containing Brineura® (cerliponase alfa) Injection, 150 mg/5 mL
1 vial of Intraventricular Electrolytes Injection, 5 mL
Package 1 of 2
Administration Kit is supplied and stored separately as package 2 of 2.
Rx Only
BioMarin®
-
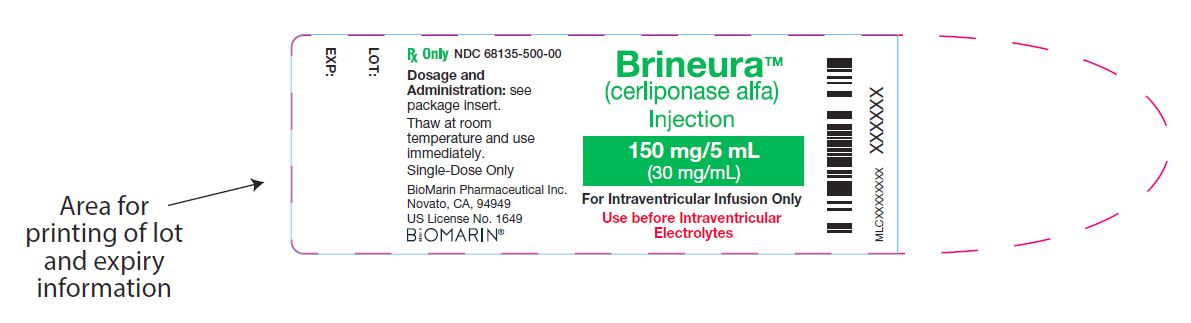
Brineura Vial
NDC: 68135-500-00
BrineuraTM
(cerliponase alfa)
Injection
150 mg/5 mL (30 mg/mL)
For Intraventricular Infusion Only
Use before Intraventricular Electrolytes
-
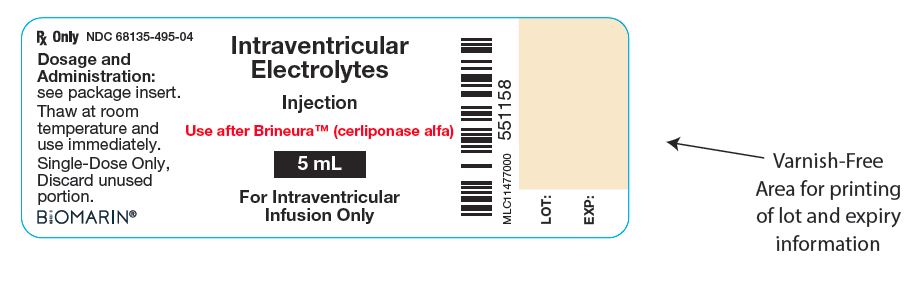
Intraventricular Electrolytes Injection Vial
NDC: 68135-495-04
Intraventricular Electrolytes
Injection
Use after BrineuraTM (cerliponase alfa)
5 mL
For Intraventricular Infusion Only
-
Administration Kit
Administration Kit for use with BrineuraTM (cerliponase alfa)
Intraventricular infusion only
Dosage and Administration: see package insert enclosed.
Contents of Administration Kit:
- Two single-use 20-mL syringes
- Two single-use syringe needles, 21 G, 25.4 mm
- One single-use extension line
- One single-use infusion set with 0.2 micron inline filter
- One single-use port needle, 22 G, 16 mm

-
INGREDIENTS AND APPEARANCE
BRINEURA
cerliponase alfa kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68135-811 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68135-811-02 1 in 1 CARTON; Type 6: Drug/Biologic Combination 04/27/2017 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 2 VIAL, GLASS 10 mL Part 2 1 VIAL, GLASS 5 mL Part 1 of 2 BRINEURA
cerliponase alfa injection, solutionProduct Information Item Code (Source) NDC: 68135-500 Route of Administration INTRAVENTRICULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CERLIPONASE ALFA (UNII: X8R2D92QP1) (CERLIPONASE ALFA - UNII:X8R2D92QP1) CERLIPONASE ALFA 150 mg in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68135-500-00 5 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761052 04/27/2017 Part 2 of 2 INTRAVENTRICULAR ELECTROLYTES
intraventricular electrolytes injection, solutionProduct Information Item Code (Source) NDC: 68135-495 Route of Administration INTRAVENTRICULAR Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68135-495-04 5 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761052 04/27/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761052 04/27/2017 Labeler - BioMarin Pharmaceutical Inc. (079722386)
Trademark Results [Brineura]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BRINEURA 87978085 5577436 Live/Registered |
Biomarin Pharmaceutical Inc. 2017-07-06 |
 BRINEURA 87518227 not registered Live/Pending |
Biomarin Pharmaceutical Inc. 2017-07-06 |
 BRINEURA 87518223 5386482 Live/Registered |
Biomarin Pharmaceutical Inc. 2017-07-06 |
 BRINEURA 86402304 5276719 Live/Registered |
Biomarin Pharmaceutical Inc. 2014-09-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.