BOTOX COSMETIC- onabotulinumtoxina injection, powder, lyophilized, for solution
BOTOX Cosmetic by
Drug Labeling and Warnings
BOTOX Cosmetic by is a Prescription medication manufactured, distributed, or labeled by Allergan, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BOTOX® Cosmetic safely and effectively. See full prescribing information for BOTOX Cosmetic.
BOTOX Cosmetic (onabotulinumtoxinA) for injection,
for intramuscular use
Initial U.S. Approval: 1989
WARNING: DISTANT SPREAD OF TOXIN EFFECT
See full prescribing information for complete boxed warning.
The effects of BOTOX Cosmetic and all botulinum toxin products may spread from the area of injection to produce symptoms consistent with botulinum toxin effects. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening and there have been reports of death. The risk of symptoms is probably greatest in children treated for spasticity but symptoms can also occur in adults, particularly in those patients who have an underlying condition that would predispose them to these symptoms. (5.2)
RECENT MAJOR CHANGES
- Warnings and Precautions, Dry Eye in Patients Treated with BOTOX Cosmetic (5.10) 11/2019
INDICATIONS AND USAGE
BOTOX Cosmetic is an acetylcholine release inhibitor and a neuromuscular blocking agent indicated in adult patients for the temporary improvement in the appearance of (1):
- Moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity
- Moderate to severe lateral canthal lines associated with orbicularis oculi activity
- Moderate to severe forehead lines associated with frontalis muscle activity
DOSAGE AND ADMINISTRATION
- Botox Cosmetic is administered by intramuscular injection
- Glabellar Lines Administration: 0.1 mL (4 Units) into each of 5 sites, for a total dose of 20 Units (2.3)
- Lateral Canthal Lines Administration: 0.1 mL (4 Units) into each of 3 sites per side (6 total injection points), for a total of 24 Units (2.3)
- Forehead Lines Administration: 0.1 mL (4 Units) into each of 5 forehead line sites (20 Units) with 0.1 mL (4 Units) into each of 5 glabellar line sites (20 Units), for a recommended total of 40 Units (2.3)
- Follow dosage and administration recommendations. In treating adults for more than one approved indications with BOTOX and BOTOX Cosmetic, do not exceed a total dose of 400 Units administered in a 3 month interval (2.1)
- See Preparation and Dilution Technique for instructions on BOTOX Cosmetic reconstitution, storage, and preparation before injection (2.2)
DOSAGE FORMS AND STRENGTHS
For Injection: 50 Units or 100 Units vacuum-dried powder in a single-dose vial for reconstitution (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Potency Units of BOTOX Cosmetic are not interchangeable with other preparations of botulinum toxin products (5.1, 11)
- Spread of toxin effects; swallowing and breathing difficulties can lead to death. Seek immediate medical attention if respiratory, speech or swallowing difficulties occur (5.2, 5.7)
- Potential serious adverse reactions after administration of BOTOX for unapproved uses (5.3)
- Adverse event reports have been received involving the cardiovascular system, some with fatal outcomes. Use caution when administering to patients with pre-existing cardiovascular disease. (5.5)
- Concomitant neuromuscular disorder may exacerbate clinical effects of treatment (5.6)
- Use with caution in patients with compromised respiratory function or dysphagia (5.7)
ADVERSE REACTIONS
The most common adverse reactions are (6.1):
- Glabellar Lines: eyelid ptosis (3%)
- Lateral Canthal Lines: eyelid edema (1%)
- Forehead Lines: headache (9%) and brow ptosis (2%)
To report SUSPECTED ADVERSE REACTIONS, contact Allergan at 1-800-678-1605 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
Patients receiving concomitant treatment of BOTOX Cosmetic and aminoglycosides or other agents interfering with neuromuscular transmission (e.g., curare-like agents), or muscle relaxants, should be observed closely because the effect of BOTOX Cosmetic may be potentiated (7)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 11/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: DISTANT SPREAD OF TOXIN EFFECT
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Instructions for Safe Use
2.2 Preparation and Dilution Technique
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Known Hypersensitivity to Botulinum Toxin
4.2 Infection at the Injection Site(s)
5 WARNINGS AND PRECAUTIONS
5.1 Lack of Interchangeability between Botulinum Toxin Products
5.2 Spread of Toxin Effect
5.3 Serious Adverse Reactions with Unapproved Use
5.4 Hypersensitivity Reactions
5.5 Cardiovascular System
5.6 Increased Risk of Clinically Significant Effects with Pre-Existing Neuromuscular Disorders
5.7 Dysphagia and Breathing Difficulties
5.8 Pre-existing Conditions at the Injection Site
5.9 Corneal Exposure and Ulceration in Patients Treated with BOTOX for Blepharospasm
5.10 Dry Eye in Patients Treated with BOTOX Cosmetic
5.11 Spatial Disorientation, Double Vision or Past-pointing in Patients Treated for Strabismus
5.12 Human Albumin and Transmission of Viral Diseases
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
6.3 Post-marketing Experience
7 DRUG INTERACTIONS
7.1 Aminoglycosides and Other Agents Interfering with Neuromuscular Transmission
7.2 Anticholinergic Drugs
7.3 Other Botulinum Neurotoxin Products
7.4 Muscle Relaxants
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Glabellar Lines
14.2 Lateral Canthal Lines
14.3 Forehead Lines
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: DISTANT SPREAD OF TOXIN EFFECT
Postmarketing reports indicate that the effects of BOTOX Cosmetic and all botulinum toxin products may spread from the area of injection to produce symptoms consistent with botulinum toxin effects. These may include asthenia, generalized muscle weakness, diplopia, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence and breathing difficulties. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening and there have been reports of death. The risk of symptoms is probably greatest in children treated for spasticity but symptoms can also occur in adults treated for spasticity and other conditions, particularly in those patients who have an underlying condition that would predispose them to these symptoms. In unapproved uses, including spasticity in children, and in approved indications, cases of spread of effect have been reported at doses comparable to those used to treat cervical dystonia and spasticity and at lower doses. [see Warnings and Precautions (5.2)]
-
1
INDICATIONS AND USAGE
BOTOX Cosmetic (onabotulinumtoxinA) is indicated in adult patients for the temporary improvement in the appearance of:
- moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity
- moderate to severe lateral canthal lines associated with orbicularis oculi activity
- moderate to severe forehead lines associated with frontalis muscle activity
- moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity
-
2
DOSAGE AND ADMINISTRATION
2.1 Instructions for Safe Use
The potency Units of BOTOX Cosmetic (onabotulinumtoxinA) for injection are specific to the preparation and assay method utilized. They are not interchangeable with other preparations of botulinum toxin products and, therefore, units of biological activity of BOTOX Cosmetic cannot be compared to nor converted into units of any other botulinum toxin products assessed with any other specific assay method [see Warnings and Precautions (5.1) and Description (11)].
Indication specific dosage and administration recommendations should be followed. In treating adult patients for one or more indications with BOTOX and BOTOX Cosmetic, the maximum cumulative dose should generally not exceed 400 Units, in a 3 month interval.
The safety and effectiveness of dosing with BOTOX Cosmetic more frequently than every 3 months have not been clinically evaluated.
The safe and effective use of BOTOX Cosmetic depends upon proper storage of the product, selection of the correct dose, and proper reconstitution and administration techniques. Physicians administering BOTOX Cosmetic must understand the relevant neuromuscular and structural anatomy of the area involved and any alterations to the anatomy due to prior surgical procedures and disease.
Do not use BOTOX Cosmetic and contact Allergan (1-800-890-4345) if:
- The carton labeling does not contain an intact seal with a translucent silver Allergan logo (on both ends of the carton) or the seal has a black circle with a diagonal line through it (i.e., prohibition sign),
- The vial label does not contain a holographic film containing the name “Allergan” within rainbow colored horizontal lines, or
- The U.S. License number 1145 is not present on the vial label and carton labeling [see How Supplied/Storage and Handling (16)]
2.2 Preparation and Dilution Technique
BOTOX Cosmetic is supplied in single-dose 50 Units and 100 Units per vial. Prior to intramuscular injection, reconstitute each vacuum-dried vial of BOTOX Cosmetic with sterile, preservative-free 0.9% Sodium Chloride Injection USP (see Table 1). Draw up the proper amount of diluent in the appropriate size needle and syringe to obtain a reconstituted solution at a concentration of 4 Units/0.1 mL and a total treatment dose of 20 Units in 0.5 mL for glabellar lines, 24 Units in 0.6 mL for lateral canthal lines, and 40 Units in 1 mL for forehead lines and glabellar lines. Then slowly inject the diluent into the vial. Discard the vial if a vacuum does not pull the diluent into the vial. Gently mix BOTOX Cosmetic with the saline by rotating the vial. Record the date and time of reconstitution on the space on the label. BOTOX Cosmetic should be administered within 24 hours after reconstitution. During this time period, reconstituted BOTOX Cosmetic should be stored in a refrigerator (2° to 8°C). BOTOX Cosmetic vials are for single-dose only. Discard any remaining solution.
Table 1: Dilution Instructions for BOTOX Cosmetic Vials (100 Units and 50 Units) Diluent* Added to
100 Unit VialResulting Dose Units per 0.1 mL Diluent* Added to
50 Unit VialResulting Dose Units per 0.1 mL 2.5 mL 4 Units 1.25 mL 4 Units *Preservative-free 0.9% Sodium Chloride Injection, USP Only
Reconstituted BOTOX Cosmetic should be clear, colorless, and free of particulate matter. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration and whenever the solution and the container permit. Do not freeze reconstituted BOTOX Cosmetic.
2.3 Administration
Draw at least 0.5 mL (for glabellar lines), 0.6 mL (for lateral canthal lines), or 1 mL (for forehead lines treated in conjunction with glabellar lines) of the properly reconstituted toxin into the sterile syringe, preferably a tuberculin syringe and expel any air bubbles in the syringe barrel. Remove the needle used to reconstitute the product and attach a 30-33 gauge needle. Confirm the patency of the needle.
Glabellar Lines
Glabellar facial lines arise from the activity of the corrugator and orbicularis oculi muscles. These muscles move the brow medially, and the procerus and depressor supercilii pull the brow inferiorly. This creates a frown or “furrowed brow”. The location, size, and use of the muscles vary markedly among individuals. Lines induced by facial expression occur perpendicular to the direction of action of contracting facial muscles. An effective dose for facial lines is determined by gross observation of the patient’s ability to activate the superficial muscles injected.
In order to reduce the complication of ptosis the following steps should be taken:
Avoid injection near the levator palpebrae superioris, particularly in patients with larger brow depressor complexes.
Lateral corrugator injections should be placed at least 1 cm above the bony supraorbital ridge.
Ensure the injected volume/dose is accurate and where feasible kept to a minimum.
Do not inject toxin closer than 1 cm above the central eyebrow.
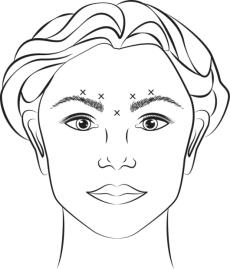
Inject 4 Units (0.1 mL) of reconstituted BOTOX Cosmetic intramuscularly into each of 5 sites, 2 in each corrugator muscle and 1 in the procerus muscle for a total dose of 20 Units (see Figure 1). Typically the initial doses of reconstituted BOTOX Cosmetic induce chemical denervation of the injected muscles one to two days after injection, increasing in intensity during the first week.
The duration of effect of BOTOX Cosmetic for glabellar lines is approximately 3-4 months.
Figure 1:
Lateral Canthal Lines
Lateral canthal lines arise largely from the activity of the orbicularis oculi muscles around the eye responsible for blinking and eyelid closure. Forceful contraction of the orbicularis oculi results in lateral and radially oriented folds (crow’s feet lines) which originate from the lateral canthus. The distribution of these radial lines differs among patients.
Injections should be given with the needle bevel tip up and oriented away from the eye. Inject 4 Units/0.1 mL of reconstituted BOTOX Cosmetic into 3 sites per side (6 total injection points) in the lateral orbicularis oculi muscle for a total of 24 Units/0.6 mL (12 Units per side). The first injection (A) should be approximately 1.5-2.0 cm temporal to the lateral canthus and just temporal to the orbital rim. If the lines in the lateral canthal region are above and below the lateral canthus, inject per Figure 2. Alternatively, if the lines in the lateral canthal region are primarily below the lateral canthus, inject per Figure 3.
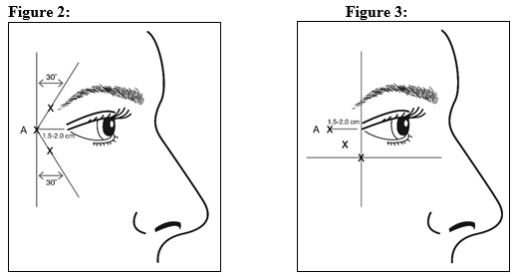
For simultaneous treatment with glabellar lines, the dose is 24 Units for lateral canthal lines and 20 Units for glabellar lines (see Glabellar Lines Administration and Figure 1), with a total dose of 44 Units.
Forehead Lines
Forehead lines arise largely from the activity of the frontalis muscles. This muscle moves the brow superiorly, interacting with the procerus, orbicularis, corrugator, and depressor supercilli. Frontalis contraction causes brow elevation. The location, size, and use of the muscles vary markedly among individuals.
Treat forehead lines in conjunction with glabellar lines (see Glabellar Lines Administration and Figure 1) to minimize the potential for brow ptosis. The recommended total dose for treatment of forehead lines (20 Units [0.5 mL]) in conjunction with glabellar lines (20 Units [0.5 mL]) is 40 Units (1mL).
When identifying the location of the appropriate injection sites in the frontalis muscle, assess the overall relationship between the size of the subject’s forehead, and the distribution of frontalis muscle activity.
Locate the following horizontal treatment rows by light palpation of the forehead at rest and maximum eyebrow elevation:
- Superior Margin of Frontalis Activity: approximately 1 cm above the most superior forehead crease
- Lower Treatment Row: midway between the superior margin of frontalis activity and the eyebrow, at least 2 cm above the eyebrow
- Upper Treatment Row: midway between the superior margin of frontalis activity and lower treatment row
Inject 4 Units (0.1 mL) of reconstituted BOTOX Cosmetic into 5 sites in the frontalis muscle, for a total of 20 Units (0.5 mL). Place the 5 injections at the intersection of the horizontal treatment rows with the following vertical landmarks (see Figure 4):
- On the lower treatment row at the midline of the face, and 0.5 – 1.5 cm medial to the palpated temporal fusion line (temporal crest); repeat for the other side.
- On the upper treatment row, midway between the lateral and medial sites on the lower treatment row; repeat for the other side.
Figure 4:
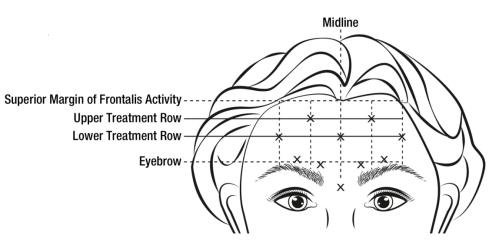
For simultaneous treatment with lateral canthal lines, the total dose is 64 Units, comprised of 20 Units for forehead lines, 20 Units for glabellar lines, and 24 Units for lateral canthal lines (see Lateral Canthal Lines Administration and Figures 2 and 3).
- The carton labeling does not contain an intact seal with a translucent silver Allergan logo (on both ends of the carton) or the seal has a black circle with a diagonal line through it (i.e., prohibition sign),
- 3 DOSAGE FORMS AND STRENGTHS
-
4
CONTRAINDICATIONS
4.1 Known Hypersensitivity to Botulinum Toxin
BOTOX Cosmetic is contraindicated in individuals with known hypersensitivity to any botulinum toxin preparation or to any of the components in the formulation [see Warnings and Precautions (5.4)].
-
5
WARNINGS AND PRECAUTIONS
5.1 Lack of Interchangeability between Botulinum Toxin Products
The potency Units of BOTOX Cosmetic are specific to the preparation and assay method utilized. They are not interchangeable with other preparations of botulinum toxin products and, therefore, units of biological activity of BOTOX Cosmetic cannot be compared to nor converted into units of any other botulinum toxin products assessed with any other specific assay method [see Description (11)].
5.2 Spread of Toxin Effect
Postmarketing safety data from BOTOX Cosmetic and other approved botulinum toxins suggest that botulinum toxin effects may, in some cases, be observed beyond the site of local injection. The symptoms are consistent with the mechanism of action of botulinum toxin and may include asthenia, generalized muscle weakness, diplopia, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence, and breathing difficulties. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening and there have been reports of death related to spread of toxin effects. The risk of symptoms is probably greatest in children treated for spasticity but symptoms can also occur in adults treated for spasticity and other conditions, and particularly in those patients who have an underlying condition that would predispose them to these symptoms. In unapproved uses, including spasticity in children, and in approved indications, symptoms consistent with spread of toxin effect have been reported at doses comparable to or lower than doses used to treat cervical dystonia and spasticity. Patients or caregivers should be advised to seek immediate medical care if swallowing, speech or respiratory disorders occur.
No definitive serious adverse event reports of distant spread of toxin effect associated with dermatologic use of BOTOX/BOTOX Cosmetic at the labeled dose of 20 Units (for glabellar lines), 24 Units (for lateral canthal lines), 40 Units (for forehead lines with glabellar lines), 44 Units (for simultaneous treatment of lateral canthal lines and glabellar lines), 64 Units (for simultaneous treatment of lateral canthal lines, glabellar lines, and forehead lines), or 100 Units (for severe primary axillary hyperhidrosis) have been reported.
No definitive serious adverse event reports of distant spread of toxin effect associated with BOTOX for blepharospasm at the recommended dose (30 Units and below), strabismus, or chronic migraine at the labeled doses have been reported.
5.3 Serious Adverse Reactions with Unapproved Use
Serious adverse reactions, including excessive weakness, dysphagia, and aspiration pneumonia, with some adverse reactions associated with fatal outcomes, have been reported in patients who received BOTOX injections for unapproved uses. In these cases, the adverse reactions were not necessarily related to distant spread of toxin, but may have resulted from the administration of BOTOX to the site of injection and/or adjacent structures. In several of the cases, patients had pre-existing dysphagia or other significant disabilities. There is insufficient information to identify factors associated with an increased risk for adverse reactions associated with the unapproved uses of BOTOX. The safety and effectiveness of BOTOX for unapproved uses have not been established.
5.4 Hypersensitivity Reactions
Serious and/or immediate hypersensitivity reactions have been reported. These reactions include anaphylaxis, serum sickness, urticaria, soft tissue edema, and dyspnea. If such a reaction occurs, further injection of BOTOX Cosmetic should be discontinued and appropriate medical therapy immediately instituted. One fatal case of anaphylaxis has been reported in which lidocaine was used as the diluent, and consequently the causal agent cannot be reliably determined.
5.5 Cardiovascular System
There have been reports following administration of BOTOX/BOTOX Cosmetic of adverse events involving the cardiovascular system, including arrhythmia and myocardial infarction, some with fatal outcomes. Some of these patients had risk factors including pre-existing cardiovascular disease. Use caution when administering to patients with pre-existing cardiovascular disease.
5.6 Increased Risk of Clinically Significant Effects with Pre-Existing Neuromuscular Disorders
Individuals with peripheral motor neuropathic diseases, amyotrophic lateral sclerosis or neuromuscular junction disorders (e.g., myasthenia gravis or Lambert-Eaton syndrome) should be monitored when given botulinum toxin. Patients with neuromuscular disorders may be at increased risk of clinically significant effects including generalized muscle weakness, diplopia, ptosis, dysphonia, dysarthria, severe dysphagia and respiratory compromise from onabotulinumtoxinA [see Warnings and Precautions (5.2, 5.7)].
5.7 Dysphagia and Breathing Difficulties
Treatment with BOTOX and other botulinum toxin products can result in swallowing or breathing difficulties. Patients with pre-existing swallowing or breathing difficulties may be more susceptible to these complications. In most cases, this is a consequence of weakening of muscles in the area of injection that are involved in breathing or oropharyngeal muscles that control swallowing or breathing [see Warnings and Precautions (5.2)].
Deaths as a complication of severe dysphagia have been reported after treatment with botulinum toxin. Dysphagia may persist for several months, and require use of a feeding tube to maintain adequate nutrition and hydration. Aspiration may result from severe dysphagia and is a particular risk when treating patients in whom swallowing or respiratory function is already compromised.
Treatment with botulinum toxins may weaken neck muscles that serve as accessory muscles of ventilation. This may result in a critical loss of breathing capacity in patients with respiratory disorders who may have become dependent upon these accessory muscles. There have been postmarketing reports of serious breathing difficulties, including respiratory failure.
Patients with smaller neck muscle mass and patients who require bilateral injections into the sternocleidomastoid muscle for the treatment of cervical dystonia have been reported to be at greater risk for dysphagia. Limiting the dose injected into the sternocleidomastoid muscle may reduce the occurrence of dysphagia. Injections into the levator scapulae may be associated with an increased risk of upper respiratory infection and dysphagia.
Patients treated with botulinum toxin may require immediate medical attention should they develop problems with swallowing, speech or respiratory disorders. These reactions can occur within hours to weeks after injection with botulinum toxin [see Warnings and Precautions (5.2)].
5.8 Pre-existing Conditions at the Injection Site
Caution should be used when BOTOX Cosmetic treatment is used in the presence of inflammation at the proposed injection site(s), ptosis, or when excessive weakness or atrophy is present in the targeted muscle(s).
5.9 Corneal Exposure and Ulceration in Patients Treated with BOTOX for Blepharospasm
Reduced blinking from injection of botulinum toxin products in or near the orbicularis oculi muscle can lead to corneal exposure, persistent corneal epithelial defect, and corneal ulceration, especially in patients with VII nerve disorders.
Vigorous treatment of any corneal epithelial defect should be employed. This may require protective drops, ointment, therapeutic soft contact lenses, or closure of the eye by patching or other means.
5.10 Dry Eye in Patients Treated with BOTOX Cosmetic
There have been reports of dry eye associated with BOTOX Cosmetic injection in or near the orbicularis oculi muscle. If symptoms of dry eye (e.g., eye irritation, photophobia, or visual changes) persist, consider referring patients to an ophthalmologist [see Warnings and Precautions (5.9)].
5.11 Spatial Disorientation, Double Vision or Past-pointing in Patients Treated for Strabismus
Inducing paralysis in one or more extraocular muscles may produce spatial disorientation, double vision or past pointing. Covering the affected eye may alleviate these symptoms.
5.12 Human Albumin and Transmission of Viral Diseases
This product contains albumin, a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases and variant Creutzfeldt-Jakob disease (vCJD). There is a theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD), but if that risk actually exists, the risk of transmission would also be considered extremely remote. No cases of transmission of viral diseases, CJD or vCJD have ever been identified for licensed albumin or albumin contained in other licensed products.
-
6
ADVERSE REACTIONS
The following adverse reactions to BOTOX Cosmetic (onabotulinumtoxinA) for injection are discussed in greater detail in other sections of the labeling:
- Spread of Toxin Effects [see Warnings and Precautions (5.2)]
- Hypersensitivity [see Contraindications (4.1) and Warnings and Precautions (5.4)]
- Dysphagia and Breathing Difficulties [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
BOTOX and BOTOX Cosmetic contain the same active ingredient in the same formulation, but have different labeled Indications and Usage. Therefore, adverse events observed with the use of BOTOX also have the potential to be observed with the use of BOTOX Cosmetic.
In general, adverse reactions occur within the first week following injection of BOTOX Cosmetic and while generally transient, may have a duration of several months or longer. Localized pain, infection, inflammation, tenderness, swelling, erythema, and/or bleeding/bruising may be associated with the injection. Needle-related pain and/or anxiety may result in vasovagal responses (including e.g., syncope, hypotension), which may require appropriate medical therapy.
Local weakness of the injected muscle(s) represents the expected pharmacological action of botulinum toxin. However, weakness of nearby muscles may also occur due to spread of toxin [see Warnings and Precautions (5.2)].
Glabellar Lines
Table 2 lists selected adverse reactions reported by >1% of BOTOX Cosmetic treated subjects (N=405) aged 18 to 75 who were evaluated in the randomized, placebo-controlled clinical studies to assess the use of BOTOX Cosmetic in the improvement of the appearance of glabellar lines.
Table 2: Adverse Reactions Reported by >1% of BOTOX Cosmetic treated Subjects and More Frequent than in Placebo-treated Subjects in Double-blind, Placebo-controlled Clinical Studies of Treatment of Glabellar Lines Adverse Reactions by System Organ Class BOTOX Cosmetic
(N=405)Placebo
(N=130)General Disorders and Administration Site Conditions
Facial pain6 (1%) 0 (0%) Nervous System Disorders
Facial paresis5 (1%) 0 (0%) Eye Disorders
Eyelid ptosis13 (3%) 0 (0%) Musculoskeletal and Connective Tissue Disorders
Muscular Weakness6 (1%) 0 (0%) Lateral Canthal Lines
Table 3 lists selected adverse reactions reported within 90 days following injection by >1% of BOTOX Cosmetic treated subjects (N=526) aged 18 to 75 who were evaluated in two randomized, double-blind, placebo-controlled clinical studies to assess the use of BOTOX Cosmetic in the improvement of the appearance of lateral canthal lines alone.
Table 3: Adverse Reaction Reported by ≥1% of BOTOX Cosmetic treated Subjects and More Frequent than in Placebo-treated Subjects Within 90 Days, in Double-blind, Placebo-controlled Clinical Studies of Treatment of Lateral Canthal Lines Adverse Reactions by System Organ Class BOTOX Cosmetic 24 Units
(N=526)Placebo
(N=530)Eye disorders
Eyelid edema
5 (1%)
0 (0%)Forehead Lines
Table 4 lists selected adverse reactions reported by >1% of BOTOX Cosmetic treated subjects (N=665) aged 18 to 77 who were evaluated in two randomized, double-blind, placebo-controlled clinical studies to assess the use of BOTOX Cosmetic in the improvement of the appearance of forehead lines with glabellar lines.
Table 4: Adverse Reactions Reported by ≥1% of BOTOX Cosmetic treated Subjects and More Frequent than in Placebo-treated Subjects, in Double-blind, Placebo-controlled Clinical Studies of Treatment of Forehead Lines Adverse Reactions by System Organ Class BOTOX Cosmetic
(20 Units forehead lines
with 20 Units glabellar lines)
(N=665)Placebo
(N=315)Nervous System Disorders
Headache58 (9%) 17 (5%) Eye Disorders
Eyelid ptosis12 (2%) 1 (0%) Skin and Subcutaneous
Tissue Disorders
Brow ptosis
Skin tightness13 (2%)
10 (2%)0 (0%)
0 (0%)There were no additional adverse drug reactions reported with the simultaneous treatment of forehead lines, glabellar lines, and lateral canthal lines.
6.2 Immunogenicity
As with all therapeutic proteins, there is a potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to BOTOX Cosmetic in the studies described below with the incidence of antibodies in other studies or to other products may be misleading. Treatment with botulinum toxins may result in the formation of neutralizing antibodies that may reduce the effectiveness of subsequent treatments by inactivating biological activity of the toxin.
In three Lateral Canthal Line trials, 916 subjects (517 subjects at 24 Units and 399 subjects at 44 Units) treated with BOTOX Cosmetic had specimens analyzed for antibody formation. Among the 916 BOTOX Cosmetic treated subjects, 14 subjects (1.5%) developed binding antibodies and no subjects (0%) developed the presence of neutralizing antibodies.
The data reflect the subjects whose test results were considered positive or negative for neutralizing activity to BOTOX Cosmetic in a mouse protection assay.
The critical factors for neutralizing antibody formation have not been well characterized. The results from some studies suggest that botulinum toxin injections at more frequent intervals or at higher doses may lead to greater incidence of antibody formation. The potential for antibody formation may be minimized by injecting with the lowest effective dose given at the longest feasible intervals between injections.
6.3 Post-marketing Experience
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
There have been spontaneous reports of death, sometimes associated with dysphagia, pneumonia, and/or other significant debility or anaphylaxis, after treatment with botulinum toxin [see Warnings and Precautions (5.4, 5.7)].
There have also been reports of adverse events involving the cardiovascular system, including arrhythmia and myocardial infarction, some with fatal outcomes. Some of these patients had risk factors including cardiovascular disease.
New onset or recurrent seizures have also been reported, typically in patients who are predisposed to experiencing these events.
The following adverse reactions by System Organ Class have been identified during post-approval use of BOTOX/BOTOX Cosmetic:
Ear and labyrinth disorders
Hypoacusis; tinnitus; vertigo
Eye disorders
Diplopia; dry eye; lagophthalmos; strabismus; visual disturbances; vision blurred
Gastrointestinal disorders
Abdominal pain; diarrhea; dry mouth; nausea; vomiting
General disorders and administration site conditions
Denervation; malaise; pyrexia
Metabolism and nutrition disorders
Anorexia
Musculoskeletal and connective tissue disorders
Localized muscle twitching/involuntary muscle contractions; muscle atrophy; myalgia
Nervous system disorders
Brachial plexopathy; dysarthria; facial palsy; hypoaesthesia; localized numbness; myasthenia gravis; paresthesia; peripheral neuropathy; radiculopathy; syncope
Respiratory, thoracic and mediastinal disorders
Aspiration pneumonia; dyspnea; respiratory depression and/or respiratory failure
Skin and subcutaneous tissue disorders
Alopecia, including madarosis; hyperhidrosis; pruritus; skin rash (including erythema multiforme, dermatitis psoriasiform, and psoriasiform eruption)
- Spread of Toxin Effects [see Warnings and Precautions (5.2)]
-
7
DRUG INTERACTIONS
No formal drug interaction studies have been conducted with BOTOX Cosmetic (onabotulinumtoxinA) for injection.
7.1 Aminoglycosides and Other Agents Interfering with Neuromuscular Transmission
Co-administration of BOTOX Cosmetic and aminoglycosides or other agents interfering with neuromuscular transmission (e.g., curare-like compounds) should only be performed with caution as the effect of the toxin may be potentiated.
7.2 Anticholinergic Drugs
Use of anticholinergic drugs after administration of BOTOX Cosmetic may potentiate systemic anticholinergic effects.
7.3 Other Botulinum Neurotoxin Products
The effect of administering different botulinum neurotoxin products at the same time or within several months of each other is unknown. Excessive neuromuscular weakness may be exacerbated by administration of another botulinum toxin prior to the resolution of the effects of a previously administered botulinum toxin.
-
8
USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no studies or adequate data from postmarketing surveillance on the developmental risk associated with use of BOTOX Cosmetic in pregnant women.
In animal studies, administrations of BOTOX Cosmetic during pregnancy resulted in adverse effects on fetal growth (decreased fetal body weight and skeletal ossification) at clinically relevant doses, which were associated with maternal toxicity [see Data].
In the U.S. general population, the estimated background risk of major birth defects and miscarriages in clinically recognized pregnancies is 2-4% and 15-20%, respectively. The background risk of major birth defects and miscarriage for the indicated populations is unknown.
Data
Animal Data
When BOTOX Cosmetic (4, 8, or 16 Units/kg) was administered intramuscularly to pregnant mice or rats two times during the period of organogenesis (on gestation days 5 and 13), reductions in fetal body weight and decreased fetal skeletal ossification were observed at the two highest doses. The no-effect dose for developmental toxicity in these studies (4 Units/kg) is approximately 4 times the average high human dose for glabellar lines, lateral canthal lines, and forehead lines of 64 Units on a body weight basis (Units/kg).
When BOTOX Cosmetic was administered intramuscularly to pregnant rats (0.125, 0.25, 0.5, 1, 4, or 8 Units/kg) or rabbits (0.063, 0.125, 0.25, or 0.5 Units/kg) daily during the period of organogenesis (total of 12 doses in rats, 13 doses in rabbits), reduced fetal body weights and decreased fetal skeletal ossification were observed at the two highest doses in rats and at the highest dose in rabbits. These doses were also associated with significant maternal toxicity, including abortions, early deliveries, and maternal death. The developmental no-effect doses in these studies of 1 Unit/kg in rats is approximately equal the average high human dose of 64 Units based on Units/kg, and the developmental no-effect dose of 0.25 Units/kg in rabbits is less than the average high human dose based on Units/kg.
When pregnant rats received single intramuscular injections (1, 4, or 16 Units/kg) at three different periods of development (prior to implantation, implantation, or organogenesis), no adverse effects on fetal development were observed. The developmental no-effect level for a single maternal dose in rats (16 Units/kg) is approximately 16 times the average high human dose of 64 Units based on Units/kg.
8.2 Lactation
Risk Summary
There are no data on the presence of BOTOX Cosmetic in human or animal milk, the effects on the breastfed child, or the effects on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for BOTOX Cosmetic and any potential adverse effects on the breastfed infant from BOTOX Cosmetic or from the underlying maternal conditions.
8.4 Pediatric Use
Safety and effectiveness in patients below the age of 18 years have not been established.
8.5 Geriatric Use
Glabellar Lines
In the two initial glabellar lines clinical studies of BOTOX Cosmetic, the responder rates appeared to be higher for subjects younger than age 65 than for subjects 65 years or older [see Clinical Studies (14)].
Lateral Canthal Lines
In the two lateral canthal lines clinical studies of BOTOX Cosmetic, the responder rates appeared to be higher for subjects younger than age 65 than for subjects 65 years or older.
Forehead Lines
In the two forehead lines clinical studies of BOTOX Cosmetic, the responder rates appeared to be higher for subjects younger than age 65 than for subjects 65 years or older.
-
10
OVERDOSAGE
Excessive doses of BOTOX Cosmetic (onabotulinumtoxinA) for injection may be expected to produce neuromuscular weakness with a variety of symptoms.
Symptoms of overdose are likely not to be present immediately following injection. Should accidental injection or oral ingestion occur or overdose be suspected, these patients should be considered for further medical evaluation and appropriate medical therapy immediately instituted, which may include hospitalization. The person should be medically supervised for several weeks for signs and symptoms of systemic muscular weakness which could be local, or distant from the site of injection [see Boxed Warning and Warnings and Precautions (5.2, 5.7)].
If the musculature of the oropharynx and esophagus are affected, aspiration may occur which may lead to development of aspiration pneumonia. If the respiratory muscles become paralyzed or sufficiently weakened, intubation and assisted respiration may be necessary until recovery takes place. Supportive care could involve the need for a tracheostomy and/or prolonged mechanical ventilation, in addition to other general supportive care.
In the event of overdose, antitoxin raised against botulinum toxin is available from the Centers for Disease Control and Prevention (CDC) in Atlanta, GA. However, the antitoxin will not reverse any botulinum toxin-induced effects already apparent by the time of antitoxin administration. In the event of suspected or actual cases of botulinum toxin poisoning, please contact your local or state Health Department to process a request for antitoxin through the CDC. If you do not receive a response within 30 minutes, please contact the CDC directly at 1-770-488-7100. More information can be obtained at http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5232a8.htm.
-
11
DESCRIPTION
BOTOX Cosmetic (onabotulinumtoxinA) for injection, is a sterile, vacuum-dried purified botulinum toxin type A, produced from fermentation of Hall strain Clostridium botulinum type A intended for intramuscular use. It is purified from the culture solution by dialysis and a series of acid precipitations to a complex consisting of the neurotoxin, and several accessory proteins. The complex is dissolved in sterile sodium chloride solution containing Albumin Human and is sterile filtered (0.2 microns) prior to filling and vacuum-drying.
The primary release procedure for BOTOX Cosmetic uses a cell-based potency assay to determine the potency relative to a reference standard. The assay is specific to Allergan’s products BOTOX and BOTOX Cosmetic. One Unit of BOTOX Cosmetic corresponds to the calculated median intraperitoneal lethal dose (LD50) in mice. Due to specific details of this assay such as the vehicle, dilution scheme and laboratory protocols, Units of biological activity of BOTOX Cosmetic cannot be compared to nor converted into Units of any other botulinum toxin or any toxin assessed with any other specific assay method. The specific activity of BOTOX Cosmetic is approximately 20 Units/nanogram of neurotoxin complex.
Each vial of BOTOX Cosmetic contains either 50 Units of Clostridium botulinum type A neurotoxin complex, 0.25 mg of Albumin Human, and 0.45 mg of sodium chloride; or 100 Units of Clostridium botulinum type A neurotoxin complex, 0.5 mg of Albumin Human, and 0.9 mg of sodium chloride in a sterile, vacuum-dried form without a preservative.
-
12
CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
BOTOX Cosmetic blocks neuromuscular transmission by binding to acceptor sites on motor nerve terminals, entering the nerve terminals, and inhibiting the release of acetylcholine. This inhibition occurs as the neurotoxin cleaves SNAP-25, a pre-synaptic protein integral to the successful docking and release of acetylcholine from vesicles situated within nerve endings. When injected intramuscularly at therapeutic doses, BOTOX Cosmetic produces partial chemical denervation of the muscle resulting in a localized reduction in muscle activity. In addition, the muscle may atrophy, axonal sprouting may occur, and extrajunctional acetylcholine receptors may develop. There is evidence that reinnervation of the muscle may occur, thus slowly reversing muscle denervation produced by BOTOX Cosmetic.
-
13
NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long term studies in animals have not been performed to evaluate carcinogenic potential of BOTOX Cosmetic.
BOTOX Cosmetic was negative in a battery of in vitro (microbial reverse mutation assay, mammalian cell mutation assay, and chromosomal aberration assay) and in vivo (micronucleus assay) genetic toxicologic assays.
In fertility studies of BOTOX Cosmetic (4, 8, or 16 Units/kg) in which either male or female rats were injected intramuscularly prior to mating and on the day of mating (3 doses, 2 weeks apart for males, 2 doses, 2 weeks apart for females) to untreated animals, reduced fertility was observed in males at the intermediate and high doses and in females at the high dose. The no-effect doses for reproductive toxicity (4 Units/kg in males, 8 Units/kg in females) are approximately 4-8 times the average high human dose for glabellar lines, lateral canthal lines, and forehead lines of 64 Units on a body weight basis (Units/kg).
-
14
CLINICAL STUDIES
14.1 Glabellar Lines
Two randomized, multi-center, double-blind, placebo-controlled studies of identical design were conducted to evaluate BOTOX Cosmetic for use in the temporary improvement of the appearance of moderate to severe glabellar facial lines. The studies enrolled healthy adults (ages 18 to 75) with glabellar lines of at least moderate severity at maximum frown. Subjects were excluded if they had ptosis, deep dermal scarring, or an inability to substantially lessen glabellar lines even by physically spreading them apart. Subjects received a single treatment with BOTOX Cosmetic (N=405, combined studies) or placebo (N=132, combined studies). Injection volume was 0.1 mL/injection site, for a dose/injection site in the active treatment groups of 4 Units. Subjects were injected intramuscularly in five sites, 1 in the procerus muscle and 2 in each corrugator supercilii muscle, for a total dose in the active treatment groups of 20 Units.
The co-primary efficacy endpoints were the investigator’s rating of glabellar line severity at maximum frown and the subject’s global assessment of change in appearance of glabellar lines, both at Day 30 post-injection. For the investigator rating, using a 4-point grading scale (0=none, 3=severe) a responder was defined as having a severity grade of 0 or 1. For the subject’s global assessment of change, the ratings were from +4 (complete improvement) to -4 (very marked worsening). A responder was defined as having a grade of at least +2 (moderate improvement). After completion of the randomized studies, subjects were offered participation in an open label, repeat treatment study to assess the safety of repeated treatment sessions.
The combined results of these two efficacy studies are presented here. The mean age was 46 years, with 32 subjects (6%) ≥65 years of age. Most of the subjects were women (82%), and Caucasian (84%). At baseline, 210 subjects (39%) had glabellar line severity scores at rest of moderate or severe.
In these studies, the severity of glabellar lines was reduced for up to 120 days in the BOTOX Cosmetic group compared to the placebo group as measured both by investigator rating of glabellar line severity at maximum frown (Table 5), and by subject’s global assessment of change in appearance of glabellar lines (Table 6).
Table 5: Investigator’s Assessment of Glabellar Line Severity at Maximum Frown – Responder Rates (% and Number of Subjects with Severity of None or Mild) Day BOTOX Cosmetic Placebo Differencea 7 74% 299/405 6% 8/132 68% (62, 74) 30b 80% 325/405 3% 4/132 77% (72, 82) 60 70% 283/403 2% 2/130 69% (64, 74) 90 48% 192/403 2% 3/128 45% (40, 51) 120 25% 102/403 2% 2/128 24% (19, 29) a 95% confidence intervals are shown in parenthesis
b Day 30: Co-Primary Efficacy Time point, p<0.001
Table 6: Subject’s Assessment of Change in Appearance of Glabellar Lines – Responder Rates (% and Number of Subjects with at Least Moderate Improvement) Day BOTOX Cosmetic Placebo Differencea 7 82% 334/405 9% 12/132 73% (68, 80) 30b 89% 362/405 7% 9/132 83% (77, 88) 60 82% 330/403 4% 5/130 78% (73, 83) 90 63% 254/403 3% 4/128 60% (54, 66) 120 39% 157/403 1% 1/128 38% (33, 43) a 95% confidence intervals are shown in parenthesis
b Day 30: Co-Primary Efficacy Time point, p<0.001
In the subset of subjects with resting severity scores of moderate or severe, the investigator assessment of a resting severity of mild or none at Day 30 was also achieved by more BOTOX Cosmetic treated subjects (74%, 119/161) than placebo treated subjects (20%, 10/49).
Analysis of the limited number of subjects 65 years or older suggested a lower treatment-associated response compared to subjects less than 65 years of age (Table 7).
Table 7: Investigator’s and Subject’s Assessment – Responder Rates for Subjects <65 and ≥65 Years of Age at Day 30 Assessment Age Group BOTOX Cosmetic (N=405) Placebo (N=132) Differencea Investigators (maximal frown) <65 83% 316/382 2% 2/123 81% (77, 86) Subjects <65 91% 346/382 7% 8/123 84% (79, 90) Investigators (maximal frown) >65 39% 9/23 22% 2/9 17% (-17, 51) Subjects >65 70% 16/23 11% 1/9 58% (31, 86) a 95% confidence intervals are shown in parenthesis
Exploratory analyses by gender suggested that responder rates in the BOTOX Cosmetic treated group were higher for women than for men for both the investigator assessment (Day 30; 85% of 334 women, 59% of 71 men) and the Subject Assessment (Day 30; 93% of women, 72% of men). In the limited number of non-Caucasian subjects (n=64 in the BOTOX Cosmetic treated group) the responder rates were similar to those observed in the Caucasian subjects.
14.2 Lateral Canthal Lines
Two multicenter, randomized, double-blind, placebo-controlled studies evaluated BOTOX Cosmetic (N=833, randomized to receive any BOTOX Cosmetic treatment or N=529 randomized to receive placebo) for the temporary improvement in the appearance of moderate to severe lateral canthal lines (LCL). Study 1 assessed BOTOX Cosmetic treatment of LCL alone; Study 2 also assessed simultaneous treatment of LCL and glabellar lines (GL). Both studies enrolled healthy adults with moderate to severe LCL at maximum smile at baseline; Study 2 also required subjects to have moderate to severe GL at maximum frown at baseline.
In the 5-month Study 1, subjects were randomized to receive a single blinded treatment of 24 Units/0.6 mL (12 Units per side) consisting of 4 Units/0.1 mL into 3 sites of each orbicularis oculi muscle with either BOTOX Cosmetic (N=222) or placebo (N=223).
In the 7-month Study 2, subjects were randomized to receive either BOTOX Cosmetic in the LCL region and placebo in the GL region (24 Units; N=306), or BOTOX Cosmetic in the LCL and GL regions (44 Units [24 Units for LCL and 20 Units for GL]; N=305), or placebo in the LCL and GL regions (0 Units; N=306). Subjects received the same 24 Units regimen for LCL as in Study 1, and the labeled 20 Units (5 injections, 4 Units per site) for GL. Subjects received the same treatment at days 1 and 120.
The primary efficacy measure was the assessment of LCL severity at maximum smile using the 4-point Facial Wrinkle Scale with Photonumeric Guide (FWS; 0=none, 1= mild, 2=moderate, 3=severe). The FWS assessment was performed independently by both investigators and subjects. The primary timepoint was day 30 following the first treatment, as compared to baseline.
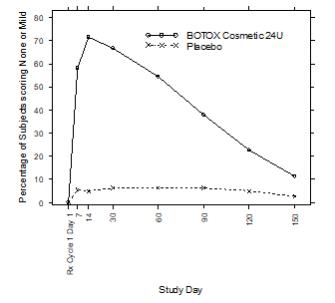
The primary efficacy response definition was a composite ≥2-grade improvement from baseline in LCL severity at maximum smile, assessed by both investigator and subject on a per-subject basis. For Studies 1 and 2, the proportion of responders was statistically significant favoring BOTOX Cosmetic (24 Units [LCL alone] and 44 Units [LCL and GL]) compared to placebo at day 30 (Table 8).
Table 8: Studies 1 and 2: Composite Investigator and Subject Assessment of LCL at Maximum Smile at Day 30 – Responder Rates (% and Number of Subjects Achieving ≥2-Grade Improvement from Baseline) Study BOTOX
Cosmetic
24 UnitsBOTOX
Cosmetic
24 Units LCL and
20 Units GLPlacebo Study 1 26.1%
58/222- 1.3%
3/223Study 2 20.3%
62/30621.3%
65/3050.0%
0/306The secondary endpoint of a responder defined as achieving a grade of none or mild for Study 1 as measured by the investigator is presented in Figure 5 below.
Figure 5: Percentage of Subjects with Treatment Success (% of Subjects achieving None or Mild from Baseline) by Visit (Study 1)
14.3 Forehead Lines
Two multicenter, randomized, double-blind, placebo-controlled studies evaluated BOTOX Cosmetic (N=921, randomized to receive any BOTOX Cosmetic treatment or N=257, randomized to receive placebo) for the temporary improvement in the appearance of moderate to severe forehead lines (FHL).
Study 1 assessed BOTOX Cosmetic treatment of FHL with glabellar lines (GL); Study 2 also assessed simultaneous treatment of FHL, GL, and lateral canthal lines [LCL]. Both studies enrolled healthy adults with moderate to severe FHL at maximum eyebrow elevation at baseline and moderate to severe GL at maximum frown at baseline; Study 2 also required subjects to have moderate to severe LCL at maximum smile at baseline.
In the 12-month Study 1, subjects were randomized to receive BOTOX Cosmetic 20 Units to the frontalis muscle with 20 Units to the glabellar region (for a total of 40 Units) or placebo in both areas.
In the 12-month Study 2, subjects were randomized to receive BOTOX Cosmetic 20 Units to the frontalis muscle, 20 Units to the glabellar region, and 0 Units to the LCL region (for a total of 40 units) or BOTOX Cosmetic 20 Units to the frontalis muscle, 20 Units to the glabellar region, and 24 Units to the LCL region (for a total of 64 Units) or placebo in all three areas.
The primary efficacy measure was the assessment of FHL severity at maximum eyebrow elevation using the 4-point Facial Wrinkle Scale with Photonumeric Guide (FWS; 0=none, 1= mild, 2=moderate, 3=severe). The FWS assessment was performed independently by both investigators and subjects. The primary timepoint was Day 30 following the first treatment.
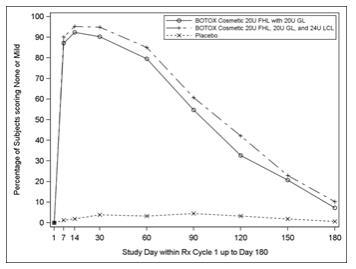
The primary efficacy response definition was a composite ≥2-grade improvement from baseline in FHL severity at maximum eyebrow elevation, assessed by both investigator and subject on a per-subject basis. For Studies 1 and 2, the proportion of responders was greater in the BOTOX Cosmetic arms compared to placebo at Day 30 (p<0.0001 for Studies 1 and 2) (Table 9).
Table 9: Studies 1 and 2: Composite Investigator and Subject Assessment of FHL Severity at Maximum Eyebrow Elevation at Day 30 – Responder Rates (% and Number of Subjects Achieving ≥2-Grade Improvement from Baseline) Study BOTOX
Cosmetic
(20 Units FHL with 20 Units GL)BOTOX
Cosmetic
(20 Units FHL,
20 Units GL,
and
24 Units LCL)Placebo Study 1 N=290
61%
- N=101
0%
Study 2 N=318
46%
N=313
53%
N=156
1%
A total of 165 and 197 subjects received 3 cycles over 1 year of BOTOX Cosmetic 40 Units (20 Units FHL with 20 Units GL) and 64 Units (20 Units FHL, 20 Units GL, and 24 Units LCL), respectively. The response rate for FHL was similar across all treatment cycles.
The results for a key secondary endpoint of responders achieving a grade of none or mild on investigator ratings at maximum eyebrow elevation of FHL severity are presented below for Studies 1 and 2.
Figure 6: Percentage of Subjects with Treatment Success (Achieving None or Mild FHL from Baseline at Maximum Eyebrow Elevation) by Visit (Study 1)
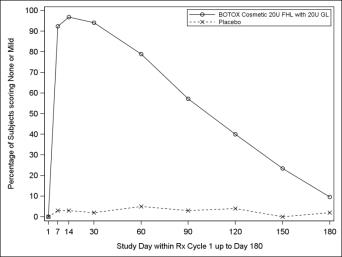
Figure 7: Percentage of Subjects with Treatment Success (Achieving None or Mild FHL from Baseline at Maximum Eyebrow Elevation) by Visit (Study 2)
The results of the Facial Line Satisfaction Questionnaire are presented in Table 10.
Table 10: Facial Lines Satisfaction Questionnaire Response Frequency at Day 60 (Percentage of Subjects) Study 1 Study 2 BOTOX
Cosmetic
(20 Units FHL with 20 Units GL)
N=289Placebo
N=99BOTOX
Cosmetic
(20 Units FHL with 20 Units GL)
N=317Placebo
N=155“Very satisfied” 57% 1% 35% 0% “Mostly satisfied” 33% 0% 47% 3% “Neither dissatisfied nor satisfied” 4%
22%
9%
23%
“Mostly dissatisfied” 4% 21% 7% 20% “Very dissatisfied” 2% 56% 2% 54% -
16
HOW SUPPLIED/STORAGE AND HANDLING
BOTOX Cosmetic (onabotulinumtoxinA) for injection is a vacuum-dried powder supplied in a single-dose vial in the following sizes:
50 Units: NDC: 0023-3919-50
100 Units: NDC: 0023-9232-01
The top and bottom flaps of the BOTOX Cosmetic cartons have a tamper-evident seal that contains a translucent silver Allergan logo, and the BOTOX Cosmetic vial labels have a holographic film that contains the name “Allergan” within rainbow colored horizontal lines (rotate the vial back and forth between your fingers under a desk lamp or fluorescent light source to see the hologram). (Note: The holographic film on the label is absent in the date/lot area.) Each BOTOX Cosmetic vial label and carton labeling also contain the U.S. License number 1145 [see Dosage and Administration (2.1)].
Do not use the product and contact Allergan for additional information at 1-800-890-4345 from 7:00 AM to 3:00 PM Pacific Time if the labeling is not as described above.
Storage
Unopened vials of BOTOX Cosmetic should be stored in a refrigerator 2° to 8°C (36º to 46ºF). Do not use after the expiration date on the vial. Reconstituted BOTOX Cosmetic should be stored in a refrigerator 2° to 8°C (36º to 46ºF) and administered within 24 hours.
-
17
PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Medication Guide)
Provide a copy of the Medication Guide and review the contents with the patient.
Swallowing, Speaking or Breathing Difficulties, or Other Unusual Symptoms
Patients should be advised to inform their doctor or pharmacist if they develop any unusual symptoms (including difficulty with swallowing, speaking, or breathing), or if any existing symptom worsens [see Boxed Warning and Warnings and Precautions (5.2, 5.7)].
Ability to Operate Machinery or Vehicles
Patients should be counseled that if loss of strength, muscle weakness, blurred vision, or drooping eyelids occur, they should avoid driving a car or engaging in other potentially hazardous activities.
Ophthalmic Adverse Reactions
Inform patients that BOTOX Cosmetic injection may cause eye dryness. Advise patients to report symptoms of eye dryness (e.g., eye pain, eye irritation, photosensitivity, or changes in vision) to their doctor.
Manufactured by: Allergan Pharmaceuticals Ireland
a subsidiary of: Allergan, Inc.
U.S. License number 1145Distributed by: Allergan USA, Inc.
Madison, NJ 07940© 2020 Allergan. All rights reserved.
All trademarks are the property of their respective owners.
Patented. See: www.allergan.com/patentsv1.0USPI3919
-
MEDICATION GUIDE
MEDICATION GUIDE
BOTOX®
BOTOX® Cosmetic
(Boe-tox)
(onabotulinumtoxinA)
for InjectionWhat is the most important information I should know about BOTOX and BOTOX Cosmetic?
BOTOX and BOTOX Cosmetic may cause serious side effects that can be life threatening, including:
-
Problems breathing or swallowing
- Spread of toxin effects
Call your doctor or get medical help right away if you have any of these problems after treatment with BOTOX or BOTOX Cosmetic:
- Problems swallowing, speaking, or breathing. These problems can happen hours, days, to weeks after an injection of BOTOX or BOTOX Cosmetic usually because the muscles that you use to breathe and swallow can become weak after the injection. Death can happen as a complication if you have severe problems with swallowing or breathing after treatment with BOTOX or BOTOX Cosmetic.
- People with certain breathing problems may need to use muscles in their neck to help them breathe. These people may be at greater risk for serious breathing problems with BOTOX or BOTOX Cosmetic.
- Swallowing problems may last for several months. People who cannot swallow well may need a feeding tube to receive food and water. If swallowing problems are severe, food or liquids may go into your lungs. People who already have swallowing or breathing problems before receiving BOTOX or BOTOX Cosmetic have the highest risk of getting these problems.
- Spread of toxin effects. In some cases, the effect of botulinum toxin may affect areas of the body away from the injection site and cause symptoms of a serious condition called botulism. The symptoms of botulism include:
- loss of strength and muscle weakness all over the body
- double vision, blurred vision and drooping eyelids
- hoarseness or change or loss of voice (dysphonia)
- trouble saying words clearly (dysarthria)
- loss of bladder control
- trouble breathing
- trouble swallowing
These problems could make it unsafe for you to drive a car or do other dangerous activities. See "What should I avoid while receiving BOTOX or BOTOX Cosmetic?"
There has not been a confirmed serious case of spread of toxin effect away from the injection site when BOTOX has been used at the recommended dose to treat chronic migraine, severe underarm sweating, blepharospasm, or strabismus, or when BOTOX Cosmetic has been used at the recommended dose to treat frown lines, crow’s feet lines, and/or forehead lines.What are BOTOX and BOTOX Cosmetic?
BOTOX is a prescription medicine that is injected into muscles and used:
- to treat overactive bladder symptoms such as a strong need to urinate with leaking or wetting accidents (urge urinary incontinence), a strong need to urinate right away (urgency), and urinating often (frequency) in adults when another type of medicine (anticholinergic) does not work well enough or cannot be taken.
- to treat leakage of urine (incontinence) in adults with overactive bladder due to neurologic disease when another type of medicine (anticholinergic) does not work well enough or cannot be taken.
- to prevent headaches in adults with chronic migraine who have 15 or more days each month with headache lasting 4 or more hours each day.
- to treat increased muscle stiffness in elbow, wrist, and finger muscles in adults with upper limb spasticity.
- to treat increased muscle stiffness in ankle and toe muscles in adults with lower limb spasticity.
- to treat increased muscle stiffness in children 2 to 17 years of age with upper limb spasticity.
- to treat increased muscle stiffness in children 2 to 17 years of age with lower limb spasticity, excluding spasticity caused by cerebral palsy.
- to treat the abnormal head position and neck pain that happens with cervical dystonia (CD) in adults.
- to treat certain types of eye muscle problems (strabismus) or abnormal spasm of the eyelids (blepharospasm) in people 12 years and older.
BOTOX Cosmetic is a prescription medicine for adults that is injected into muscles and used for a short period of time (temporary) to improve the look of:
- moderate to severe frown lines between the eyebrows (glabellar lines)
- moderate to severe crow’s feet lines
- moderate to severe forehead lines
It is not known whether BOTOX is safe or effective in people younger than:
- 18 years of age for treatment of urinary incontinence
- 18 years of age for treatment of chronic migraine
- 16 years of age for treatment of cervical dystonia
- 18 years of age for treatment of hyperhidrosis
- 12 years of age for treatment of strabismus or blepharospasm
- 2 years of age for treatment of spasticity
It is not known whether BOTOX and BOTOX Cosmetic are safe or effective to prevent headaches in people with migraine who have 14 or fewer headache days each month (episodic migraine).
It is not known whether BOTOX and BOTOX Cosmetic are safe or effective for other types of muscle spasms or for severe sweating anywhere other than your armpits.
It is not known if BOTOX Cosmetic is safe and effective for use more than 1 time every 3 months.Who should not receive BOTOX or BOTOX Cosmetic?
Do not receive BOTOX or BOTOX Cosmetic if you:- are allergic to any of the ingredients in BOTOX or BOTOX Cosmetic. See the end of this Medication Guide for a list of ingredients in BOTOX and BOTOX Cosmetic.
- had an allergic reaction to any other botulinum toxin product such as Myobloc®, Dysport®, or Xeomin®
- have a skin infection at the planned injection site
- are being treated for urinary incontinence and have a urinary tract infection (UTI)
- are being treated for urinary incontinence and find that you cannot empty your bladder on your own (only applies to people who are not routinely catheterizing)
What should I tell my doctor before receiving BOTOX or BOTOX Cosmetic?
Tell your doctor about all your medical conditions, including if you:
- have a disease that affects your muscles and nerves (such as amyotrophic lateral sclerosis [ALS or Lou Gehrig's disease], myasthenia gravis or Lambert-Eaton syndrome). See "What is the most important information I should know about BOTOX and BOTOX Cosmetic?"
- have allergies to any botulinum toxin product
- had any side effect from any botulinum toxin product in the past
- have or have had a breathing problem, such as asthma or emphysema
- have or have had swallowing problems
- have or have had bleeding problems
- have plans to have surgery
- had surgery on your face
- have weakness of your forehead muscles, such as trouble raising your eyebrows
- have drooping eyelids
- have any other change in the way your face normally looks
- have symptoms of a urinary tract infection (UTI) and are being treated for urinary incontinence. Symptoms of a urinary tract infection may include pain or burning with urination, frequent urination, or fever.
- have problems emptying your bladder on your own and are being treated for urinary incontinence
- are pregnant or plan to become pregnant. It is not known if BOTOX or BOTOX Cosmetic can harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if BOTOX or BOTOX Cosmetic passes into breast milk.
Using BOTOX or BOTOX Cosmetic with certain other medicines may cause serious side effects. Do not start any new medicines until you have told your doctor that you have received BOTOX or BOTOX Cosmetic in the past.
Especially tell your doctor if you:
- have received any other botulinum toxin product in the last four months
- have received injections of botulinum toxin, such as Myobloc® (rimabotulinumtoxinB), Dysport® (abobotulinumtoxinA), or Xeomin® (incobotulinumtoxinA) in the past. Be sure your doctor knows exactly which product you received.
- have recently received an antibiotic by injection
- take muscle relaxants
- take an allergy or cold medicine
- take a sleep medicine
- take anti-platelets (aspirin-like products) and/or anti-coagulants (blood thinners)
Know the medicines you take. Keep a list of your medicines with you to show your doctor and pharmacist each time you get a new medicine.How will I receive BOTOX or BOTOX Cosmetic? -
BOTOX or BOTOX Cosmetic is an injection that your doctor will give you.
-
BOTOX is injected into your affected muscles, skin, or bladder.
-
BOTOX Cosmetic is injected into your affected muscles.
- Your doctor may change your dose of BOTOX or BOTOX Cosmetic, until you and your doctor find the best dose for you.
- Your doctor will tell you how often you will receive your dose of BOTOX or BOTOX Cosmetic injections.
What should I avoid while receiving BOTOX or BOTOX Cosmetic?
BOTOX and BOTOX Cosmetic may cause loss of strength or general muscle weakness, vision problems, or dizziness within hours to weeks of taking BOTOX or BOTOX Cosmetic. If this happens, do not drive a car, operate machinery, or do other dangerous activities. See "What is the most important information I should know about BOTOX and BOTOX Cosmetic?"What are the possible side effects of BOTOX and BOTOX Cosmetic?
BOTOX and BOTOX Cosmetic can cause serious side effects. See "What is the most important information I should know about BOTOX and BOTOX Cosmetic?"
Other side effects of BOTOX and BOTOX Cosmetic include:
- dry mouth
- discomfort or pain at the injection site
- tiredness
- headache
- neck pain
- eye problems: double vision, blurred vision, decreased eyesight, drooping eyelids, swelling of your eyelids, and dry eyes.
- drooping eyebrows
- urinary tract infection in people being treated for urinary incontinence
- painful urination in people being treated for urinary incontinence
- inability to empty your bladder on your own and are being treated for urinary incontinence. If you have difficulty fully emptying your bladder after getting BOTOX, you may need to use disposable self-catheters to empty your bladder up to a few times each day until your bladder is able to start emptying again.
- allergic reactions. Symptoms of an allergic reaction to BOTOX or BOTOX Cosmetic may include: itching, rash, red itchy welts, wheezing, asthma symptoms, or dizziness or feeling faint. Tell your doctor or get medical help right away if you are wheezing or have asthma symptoms, or if you become dizzy or faint.
- upper respiratory tract infection
These are not all the possible side effects of BOTOX and BOTOX Cosmetic. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.General information about BOTOX and BOTOX Cosmetic:
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. This Medication Guide summarizes the most important information about BOTOX and BOTOX Cosmetic. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about BOTOX and BOTOX Cosmetic that is written for healthcare professionals.What are the ingredients in BOTOX and BOTOX Cosmetic?
Active ingredient: onabotulinumtoxin A
Inactive ingredients: human albumin and sodium chlorideManufactured by: Allergan Pharmaceuticals Ireland a subsidiary of: Allergan, Inc. 2525 Dupont Dr. Irvine, CA 92612
©2019 Allergan. All rights reserved.
All trademarks are the property of their respective owners.
Patented. See: www.allergan.com/patents

This Medication Guide has been approved by the U.S. Food and Drug Administration Revised: 10/2019
v3.0MG1145
-
Problems breathing or swallowing
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BOTOX COSMETIC
onabotulinumtoxina injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0023-3919 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BOTULINUM TOXIN TYPE A (UNII: E211KPY694) (BOTULINUM TOXIN TYPE A - UNII:E211KPY694) BOTULINUM TOXIN TYPE A 50 [USP'U] Inactive Ingredients Ingredient Name Strength ALBUMIN HUMAN (UNII: ZIF514RVZR) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0023-3919-50 1 in 1 CARTON 07/15/2008 1 1 in 1 VIAL, GLASS; Type 0: Not a Combination Product 2 NDC: 0023-3919-51 1 in 1 CARTON 07/15/2008 2 1 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103000 07/15/2008 BOTOX COSMETIC
onabotulinumtoxina injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0023-9232 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BOTULINUM TOXIN TYPE A (UNII: E211KPY694) (BOTULINUM TOXIN TYPE A - UNII:E211KPY694) BOTULINUM TOXIN TYPE A 100 [USP'U] Inactive Ingredients Ingredient Name Strength ALBUMIN HUMAN (UNII: ZIF514RVZR) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0023-9232-02 1 in 1 CARTON 05/20/2008 1 1 in 1 VIAL, GLASS; Type 0: Not a Combination Product 2 NDC: 0023-9232-01 1 in 1 CARTON 05/20/2008 2 1 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103000 05/20/2008 Labeler - Allergan, Inc. (144796497)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.