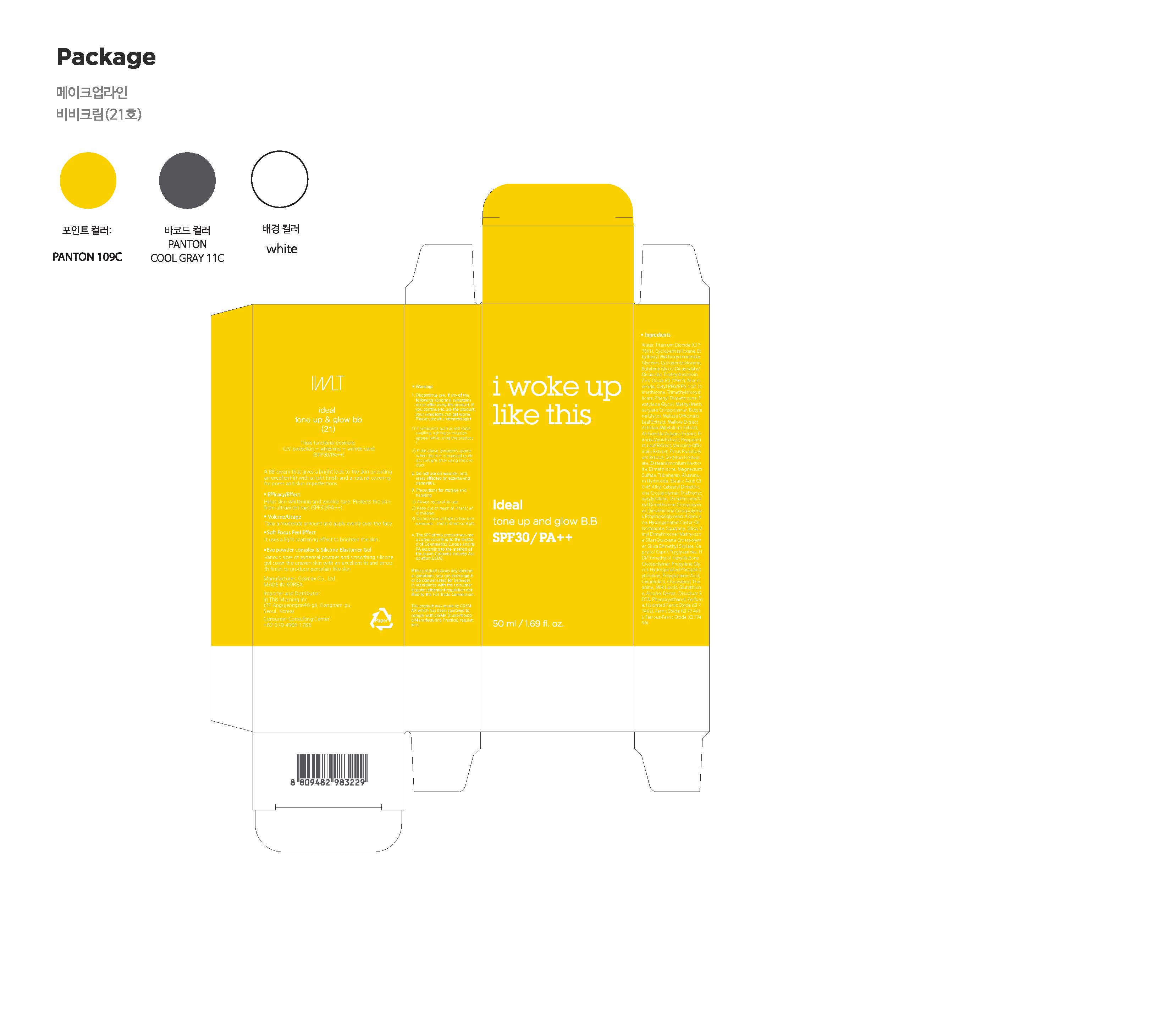
IDEAL TONE UP GLOW BB by In This Morning Drug Facts
IDEAL TONE UP GLOW BB by
Drug Labeling and Warnings
IDEAL TONE UP GLOW BB by is a Otc medication manufactured, distributed, or labeled by In This Morning. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
IDEAL TONE UP GLOW BB- titanium dioxide, zinc oxide, niacinamide, adenosine cream
In This Morning
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Drug Facts
Water, Cyclopentasiloxane, Ethylhexyl Methoxycinnamate, Glycerin, Cyclopentasiloxane, Butylene Glycol Dicaprylate/Dicaprate, Triethylhexanoin, Cetyl PEG/PPG-10/1 Dimethicone, Trimethylsiloxysilicate, Phenyl Trimethicone, Pentylene Glycol, Methyl Methacrylate Crosspolymer, Butylene Glycol, Melissa Officinalis Leaf Extract, Mallow Extract, Achillea Millefolium Extract, Alchemilla Vulgaris Extract, Primula Veris Extract, Peppermint Leaf Extract, Veronica Officinalis Extract, Pinus Pumilio Bark Extract, Sorbitan Isostearate, Disteardimonium Hectorite, Dimethicone, Magnesium Sulfate, Tribehenin, Aluminum Hydroxide, Stearic Acid, C30-45 Alkyl Cetearyl Dimethicone Crosspolymer, Triethoxycaprylylsilane, Dimethicone/Vinyl Dimethicone Crosspolymer, Dimethicone Crosspolymer, Ethylhexylglycerin, Hydrogenated Castor Oil Isostearate, Squalane, Silica, Vinyl Dimethicone/ Methyicone SilsesQuioxane Crosspolymer, Silica Dimethyl Silylate, Caprylic/ Capric Tryglycerides, HDI/Trimethylol Hexyllactone Crosspolymer, Propylene Glycol, Hydrogenated Phospatidylcholine, Polyglutamic Acid, Ceramide 3, Cholesterol, Theanine, Milk Lipids, Glutathione, Alcohol Denat., Disodium EDTA, Phenoxyethanol, Perfume, Hydrated Ferric Oxide (CI77492), Ferric Oxide (CI 77 491), Ferrous-Ferric Oxide (CI 77499)
1. Do not use in the following cases(Eczema and scalp wounds)
2.Side Effects
1)Due to the use of this druf if rash, irritation, itching and symptopms of hypersnesitivity occur dicontinue use and consult your phamacisr or doctor
3.General Precautions
1)If in contact with the eyes, wash out thoroughty with water If the symptoms are servere, seek medical advice immediately
2)This product is for exeternal use only. Do not use for internal use
4.Storage and handling precautions
1)If possible, avoid direct sunlight and store in cool and area of low humidity
2)In order to maintain the quality of the product and avoid misuse
3)Avoid placing the product near fire and store out in reach of children
| IDEAL TONE UP GLOW BB
titanium dioxide, zinc oxide, niacinamide, adenosine cream |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - In This Morning (694519423) |
| Registrant - In This Morning (694519423) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| In This Morning | 694519423 | manufacture(71217-0009) , label(71217-0009) , pack(71217-0009) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.