NEXGARD COMBO- esafoxolaner, eprinomectin, and praziquantel solution
NexGard COMBO by
Drug Labeling and Warnings
NexGard COMBO by is a Animal medication manufactured, distributed, or labeled by Boehringer Ingelheim Animal Health USA Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Caution:
-
Description:
NexGard® COMBO is a topical solution containing esafoxolaner, eprinomectin and praziquantel available in 0.3 mL and 0.9 mL unit applicators to treat cats from 1.8 lbs to 33 lbs. Each mL of NexGard® COMBO contains 12 mg of esafoxolaner, 4 mg of eprinomectin, and 83 mg of praziquantel. Inactive ingredients: dimethyl isosorbide, unstabilized glycerol formal, and butylated hydroxytoluene.
Esafoxolaner is a member of the aryl isoxazoline class of compounds. Its chemical name is 4-[(5S)-5-[3-chloro-5-(trifluoromethyl)phenyl]-5-(trifluoromethyl)-4, 5-dihydro-1,2-oxazol-3-yl]-N-{2-oxo-2-[(2,2,2-trifluoroethyl)amino]ethyl}-1-naphthamide.
Eprinomectin belongs to the avermectin class of anthelmintics and is a mixture of homologous components referred to as eprinomectin B1a and B1b. The chemical name for eprinomectin B1a is (4”R)-acetylamino-5-O-demethyl-4”-deoxyavermectin A1a. The chemical name for eprinomectin B1b is (4”R)-acetylamino-5-O-demethyl-25-de(1-methylpropyl)- 4”-deoxy-25-(1-methylethyl)avermectin A1a.
Praziquantel is a pyrazinoisoquinoline anthelmintic. Its chemical name is 2-(Cyclohexylcarbonyl)- 1,2,3,6, 7,11b-hexahydro-4H-pyrazino[2,1-a]-isoquinolin-4-one.
-
Indications:
NexGard® COMBO is indicated for the prevention of heartworm disease caused by Dirofilaria immitis and for the treatment and control of roundworm (fourth stage larval and adult Toxocara cati), hookworm (fourth stage larval and adult Ancylostoma tubaeforme; adult Ancylostomabraziliense), and tapeworm (Dipylidium caninum) infections. NexGard® COMBO kills adult fleas (Ctenocephalides felis) and is indicated for the treatment and prevention of flea infestations and the treatment and control of Ixodes scapularis (black-legged tick) and Amblyomma americanum (lone star tick) infestations for one month in cats and kittens 8 weeks of age and older, and weighing 1.8 lbs or greater.
-
Dosage and Administration:
NexGard® COMBO is dosed at a minimum of 0.055 mL/lb (0.12 mL/kg), which delivers a minimum dose of 0.65 mg/lb (1.44 mg/kg) esafoxolaner, 0.22 mg/lb (0.48 mg/kg) eprinomectin, and 4.53 mg/lb (9.98 mg/kg) praziquantel.
For heartworm disease prevention, apply once monthly for at least three months after last exposure to mosquitoes (see Effectiveness).
Administer the entire contents of a NexGard® COMBO unit applicator topically once a month as specified in the following table:
Dosing Schedule
- Cat Weight (lb)
- Volume (mL)
- Esafoxolaner (mg)
- Eprinomectin (mg)
- Praziquantel (mg)
- 1.8- 5.5
- 0.3
3.6
1.2
- 24.9
- 5.6-16.5
- 0.9
10.8
3.6
- 74.7
- 16.6-22
- 0.3 + 0.9
14.4
4.8
- 99.6
- 22.1-33
- 0.9 + 0.9
21.6
7.2
- 149.4
A veterinarian or veterinary technician should demonstrate or instruct the pet owner regarding the appropriate technique for applying NexGard® COMBO topically to cats and kittens prior to first use.
Keep product in original packaging until ready to use.
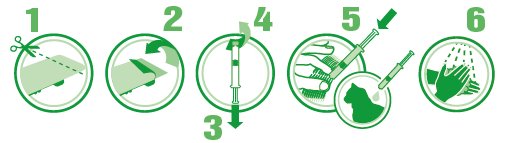
- 1. Use scissors to cut the blister along the dotted line.
- 2. Then pull the lid away.
- 3. Remove the applicator from the package and hold it upright. Pull back the plunger slightly.
- 4. Twist and pull off the cap.
- 5. Part the hair on the midline of the neck, between the base of the skull and the shoulder blades until the skin is visible. Place the tip of the applicator on the skin and apply the entire contents directly onto the skin in one spot. The product should be applied to dry skin on an area where the cat cannot lick it off. If the weight of the cat requires a second application, apply the contents in the same manner as described above in the same location.
- 6. Wash hands after use with soap and water.
Heartworm Prevention:
For the prevention of heartworm disease, NexGard® COMBO should be administered once a month year-round. At a minimum, administration of NexGard® COMBO should start at least 1 month before the cat’s first expected exposure to mosquitoes and monthly thereafter until at least 3 months after the cat’s last seasonal exposure to mosquitoes (see Effectiveness). If a dose is missed and a 30-day interval between doses is exceeded, administer NexGard® COMBO immediately and resume the monthly dosing schedule. Treatment with fewer than 3 monthly doses may not provide complete heartworm prevention. When replacing another monthly heartworm preventive product in a heartworm prevention program, the first treatment with NexGard® COMBO should be given within one month of the last dose of the former medication. At the discretion of the veterinarian, cats older than 6 months of age may be tested to determine the presence of existing heartworm infection before treatment with NexGard® COMBO. Cats already infected with adult heartworms can be given NexGard® COMBO monthly to prevent further infections.
Flea Treatment and Prevention:
For the treatment and prevention of flea infestations, the use of NexGard® COMBO may begin at any time of year. NexGard® COMBO should be administered year-round at monthly intervals or begin at least one month before fleas become active. However, an environmental infestation may persist for a short time after beginning treatment with NexGard® COMBO because of the development of adult fleas from eggs that were laid prior to the initiation of treatment.
Tick Treatment and Control:
For the treatment and control of infestations with Ixodes scapularis and Amblyomma americanum, the use of NexGard® COMBO may begin at any time of year. NexGard® COMBO should be administered year-round at monthly intervals or begin at least one month before the ticks become active.
Treatment and Control of Roundworms, Hookworms, and Tapeworms:
NexGard® COMBO provides treatment and control of roundworms (adult and fourth stage larval Toxocara cati), hookworms (adult and fourth stage larval Ancylostoma tubaeforme; adult Ancylostoma braziliense), and tapeworms (Dipylidium caninum). For the treatment of hookworm, roundworms and tapeworm infections, NexGard® COMBO should be administered once as a single dose. Monthly use of NexGard® COMBO will control any subsequent infections. Cats may be exposed to and can become infected with roundworms, hookworms, and tapeworms throughout the year, regardless of season or climate.
- Contraindications:
-
Human Warnings:
Not for human use. Keep this and all drugs out of sight and reach of children.
Avoid direct contact with application site for 4 hours or until visibly dry.
This product may act as a mild to moderate eye irritant.
Keep product in the original packaging until use. Wash hands after product administration. If the product accidentally gets into the eyes, rinse thoroughly with water. If wearing contact lenses, flush the eyes first with water and then remove the lenses and continue to flush thoroughly with water. In case of accidental ingestion, or if skin or eye irritation occurs, contact a poison control center or physician for treatment advice.
-
Precautions:
Esafoxolaner, one of the ingredients in NexGard® COMBO, is a member of the isoxazoline class. This class has been associated with neurologic adverse reactions including tremors, ataxia, and seizures. Seizures have been reported in cats receiving isoxazoline class drugs, even in cats without a history of seizures. Use with caution in cats with a history of seizures or neurologic disorders.
Do not administer orally. Cats may salivate excessively if NexGard® COMBO is accidentally administered orally or is ingested through licking/grooming the application site (see Target Animal Safety).
The safety of NexGard® COMBO has not been fully evaluated in breeding, pregnant, or lactating cats.
The safety of NexGard® COMBO has not been tested in kittens less than 8 weeks of age or weighing less than 1.8 lbs (0.8 kg).
-
Adverse Reactions:
In a field safety and effectiveness study, which included a total of 201 households and 380 treated cats (244 cats treated with NexGard® COMBO, 136 cats treated with an active control), the safety of NexGard® COMBO was evaluated over a 90-day period through in-clinic physical examinations or through reporting of abnormalities by the owner. The most frequently reported reactions in the NexGard® COMBO and active control groups are presented in the following table.
Adverse Reactions by Treatment Group
- EVENT
- Treatment Group
- NEXGARD® COMBO
- Active Control
- n1
- % (n=244)
- n2
- % (n=136)
- Vomiting
- 16
- 6.56
- 8
5.88
Application Site Hair Change
- 9
- 3.69
- 0
0.00
- Anorexia
- 7
- 2.87
- 4
2.94
- Lethargy
- 6
- 2.46
- 5
3.68
- Bacterial skin infection
- 4
- 1.64
- 1
0.74
- Itching
- 4
- 1.64
- 0
0.00
- Sneezing
- 4
- 1.64
- 5
3.68
- Skin Peeling
- 3
- 1.23
- 2
1.47
- Diarrhea
- 3
- 1.23
- 3
2.21
- Epiphora
- 3
- 1.23
- 1
0.74
- Hypersalivation
- 3
- 1.23
- 0
0.00
- Hyperthermia
- 3
- 1.23
- 0
0.00
- Alopecia
- 2
- 0.82
- 0
0.00
- Dermal thickening
- 2
- 0.82
- 0
0.00
- Ear Pruritus
- 2
- 0.82
- 1
0.74
- Application Site Redness
- 2
- 0.82
- 0
0.00
- Conjunctivitis
- 1
- 0.41
- 1
0.74
1Number of cats treated with NexGard® COMBO with the identified abnormality.
2Number of cats treated with Active Control with the identified abnormality.
-
Contact Information:
To report suspected adverse events, for technical assistance or to obtain a copy of the SDS, contact Boehringer Ingelheim Animal Health USA Inc. at 1-888-637-4251 or www.nexgardforpets.com.
For additional information about reporting adverse drug experiences for animal drugs, contact FDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae.
The Safety Data Sheet (SDS) provides additional occupational safety information. For customer service or to obtain product information, including the SDS, call 1-888-637-4251.
-
Clinical Pharmacology:
Mode of Action:
Esafoxolaner is a member of the isoxazoline family, shown to bind at a site to inhibit insect and acarine ligand-gated chloride channels, in particular those gated by the neurotransmitter gamma-aminobutyric acid (GABA), thereby blocking pre- and post-synaptic transfer of chloride ions across cell membranes. Prolonged esafoxolaner-induced hyperexcitation results in uncontrolled activity of the central nervous system and death of insects and acarines. The selective toxicity of esafoxolaner between insects/acarines and mammals may be inferred by the differential sensitivity of the insects/acarines’ GABA receptors versus mammalian GABA receptors.
Eprinomectin is an endectocide in the macrocyclic lactone class that binds to glutamate gated chloride channels that are present in invertebrate nerve and muscle cells and increases the permeability of the cell membrane to chloride ions that triggers hyperpolarization of the nerve or muscle cell in susceptible parasites, resulting in paralysis and death of the parasite.
Praziquantel’s mode of action is not precisely known, but treated tapeworms undergo muscular paralysis accompanied by a rapid influx of calcium ions and the disruption of the tegument.
Pharmacokinetics:
After a single topical administration to healthy male and female cats of a combined topical formulation containing esafoxolaner (12 mg/mL), eprinomectin (4 mg/mL), and praziquantel (83 mg/mL), at dose volumes of 0.06, 0.12, or 0.24 mL/kg, there was a dose proportional increase in the exposure of each ingredient based on maximum plasma concentration (Cmax) and area under the plasma concentration time curve (AUC). After repeated monthly doses of the combined topical formulation at the target dose of 1.44 mg/kg esafoxolaner, 0.48 mg/kg eprinomectin, and 9.98 mg/kg praziquantel, steady state was reached by the fourth dose for esafoxolaner and after the second dose for eprinomectin and praziquantel. Additionally, modest accumulation was observed for esafoxolaner (approximately 3-fold) and praziquantel (approximately 1.5- to 2-fold) between the first and fifth dose, whereas no accumulation was observed for eprinomectin.
-
Effectiveness:
Heartworm Prevention:
In well-controlled laboratory studies, NexGard® COMBO (esafoxolaner, eprinomectin, and praziquantel topical solution) was 100% effective in preventing the development of heartworms in cats inoculated with infective larvae of Dirofilaria immitis 30 days prior to the first of three consecutive monthly treatments.
Flea Treatment and Prevention:
In a well-controlled laboratory study, NexGard® COMBO killed >92% of fleas within 24 hours. During subsequent weekly infestations, NexGard® COMBO killed ≥95.5% of fleas within 24 hours through Day 31 and killed fleas before they could lay eggs. The effectiveness against adult fleas at 24 hours post-infestation in the treated cats virtually eliminated flea egg production (99.8 – 100% control of flea egg production by 24 hours) throughout the remainder of the month. In a field safety and effectiveness study in the United States, conducted in households with existing flea infestations, the effectiveness of NexGard® COMBO against fleas was 97.8%, 99.6%, and 99.9% when assessed on Days 30, 60, and 90, respectively. Cats with signs of flea allergy dermatitis showed improvement in alopecia, dermatitis/pyodermatitis, pruritus, erythema, papules, and scaling, as a direct result of eliminating fleas.
Tick Treatment and Control:
In well-controlled laboratory studies, NexGard® COMBO demonstrated ≥95.1% effectiveness against Ixodes scapularis 48 hours post-infestation for a month and ≥95.6% effectiveness against Amblyomma americanum 72 hours post-infestation for a month.
Treatment and Control of Roundworms, Hookworms, and Tapeworms:
In 2 well-controlled laboratory studies, NexGard® COMBO provided 98.9% and 100% effectiveness against natural and/or induced roundworm infections with the dose-limiting gastrointestinal nematode species (adult Toxocara cati), respectively.
Effectiveness studies against fourth stage larval Toxocara cati and hookworms (adult and fourth stage larval Ancylostoma tubaeforme; adult Ancylostoma braziliense) were conducted with an early formulation. The doses of eprinomectin in this early formulation are equivalent to that of the final formulation of NexGard® COMBO.
In well-controlled laboratory studies, NexGard® COMBO provided on average 92.8% effectiveness against natural and/or induced infections with Dipylidium caninum.
-
Target Animal Safety:
Margin of Safety Study:
NexGard® COMBO was applied topically to healthy kittens (8 to 9 weeks of age) at 1X, 3X, or 5X the maximum exposure dose six times at 28-day intervals; kittens in the control group were dosed with mineral oil. One kitten in the 5X group exhibited recumbency, tremors, hypothermia, ataxia, disorientation, and pupil dilation (responsive to light) 9 hours after the third dose. This kitten received supportive care, including washing the application site, and recovered within 48 hours post-dose. During necropsy, a dark red subcutaneous area (≤5mm diameter) was observed in the treatment site area of three cats in the 5X group, but microscopic examination revealed no histologic abnormalities. No significant changes related to NexGard® COMBO were observed for physical examination, body weight, clinical pathology (hematology, coagulation, and serum chemistry), histopathology, or organ weights.
Study in Heartworm Positive Cats:
Adult cats, 4.7 to 6.6 months of age, were experimentally infected with adult heartworms (D. immitis) by venous transplantation. All cats were negative for heartworm antibody, antigen and microfilariae prior to transplantation. Two weeks after transplantation, immunoserology verified positive antigen and the presence of microfilariae in all enrolled cats. A combination of fipronil, eprinomectin, praziquantel, and (S)-methoprene was applied topically to cats at 1X or 3X the maximum exposure dose once every 28 days for three consecutive treatments; cats in the control group were dosed with mineral oil. One cat in the 1X group exhibited cyanotic mucous membranes and tachypnea for 24 hours following the first treatment. The cat recovered and exhibited no abnormal signs following two subsequent treatments. There was no difference between the treatment groups in the number of adult D. immitis recovered at the end of the study.
Oral Administration Study:
Oral tolerance was evaluated to assess the effects of accidental oral ingestion. Kittens (male and female) ranging in age from 7.4 to 8.9 weeks were orally administered NexGard® COMBO at 1X the maximum exposure dose; kittens in the control group were dosed with saline. Cats were observed for adverse reactions at 1, 2, 3, 4, and 8 hours following administration, then twice a day until Day 14. All 8 cats administered NexGard® COMBO immediately exhibited excessive hypersalivation after oral administration. However, all cats stopped salivating within 1 hour after exposure. No additional health-related observations were seen for the remainder of the study.
- How Supplied:
- Storage Information:
-
SPL UNCLASSIFIED SECTION
Approved by FDA under NADA # 141-570
Marketed by:
Boehringer Ingelheim Animal Health USA Inc.
Duluth, GA 30096
NEXGARD® is a registered trademark and NEXGARD COMBO is a trademark of Boehringer Ingelheim Animal Health France, used under license.
©2023 Boehringer Ingelheim Animal Health USA Inc. All rights reserved.
184238-003 Rev. 04/2023
-
Principal Display Panel – 0.3 mL Display Carton
FOR CATS 1.8 – 5.5 LBS 3 Applicators 0.3 mL
NexGard® COMBO
(esafoxolaner, eprinomectin, and praziquantel topical solution)
Apply once a month. Topical solution for cats and kittens 8 weeks of age and older.
Each applicator contains 3.6 mg esafoxolaner, 1.2 mg eprinomectin, and 24.9 mg praziquantel.
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Approved by FDA under NADA # 141-570
-
Principal Display Panel – 0.9 mL Display Carton
FOR CATS 5.6 – 16.5 LBS 3 Applicators 0.9 mL
NexGard® COMBO
(esafoxolaner, eprinomectin, and praziquantel topical solution)
Apply once a month. Topical solution for cats and kittens 8 weeks of age and older.
Each applicator contains 10.8 mg esafoxolaner, 3.6 mg eprinomectin, and 74.7 mg praziquantel.
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Approved by FDA under NADA # 141-570
-
INGREDIENTS AND APPEARANCE
NEXGARD COMBO
esafoxolaner, eprinomectin, and praziquantel solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 0010-4120 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ESAFOXOLANER (UNII: 46658IQN7V) (ESAFOXOLANER - UNII:46658IQN7V) ESAFOXOLANER 12 mg in 1 mL EPRINOMECTIN (UNII: 75KP30FD8O) (EPRINOMECTIN - UNII:75KP30FD8O) EPRINOMECTIN 4 mg in 1 mL PRAZIQUANTEL (UNII: 6490C9U457) (PRAZIQUANTEL - UNII:6490C9U457) PRAZIQUANTEL 83 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0010-4120-01 1 in 1 CARTON 1 0.3 mL in 1 APPLICATOR 2 NDC: 0010-4120-02 3 in 1 CARTON 2 0.3 mL in 1 APPLICATOR 3 NDC: 0010-4120-03 6 in 1 CARTON 3 0.3 mL in 1 APPLICATOR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141570 05/10/2023 NEXGARD COMBO
esafoxolaner, eprinomectin, and praziquantel solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 0010-4121 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ESAFOXOLANER (UNII: 46658IQN7V) (ESAFOXOLANER - UNII:46658IQN7V) ESAFOXOLANER 12 mg in 1 mL EPRINOMECTIN (UNII: 75KP30FD8O) (EPRINOMECTIN - UNII:75KP30FD8O) EPRINOMECTIN 4 mg in 1 mL PRAZIQUANTEL (UNII: 6490C9U457) (PRAZIQUANTEL - UNII:6490C9U457) PRAZIQUANTEL 83 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0010-4121-01 1 in 1 CARTON 1 0.9 mL in 1 APPLICATOR 2 NDC: 0010-4121-02 3 in 1 CARTON 2 0.9 mL in 1 APPLICATOR 3 NDC: 0010-4121-03 6 in 1 CARTON 3 0.9 mL in 1 APPLICATOR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141570 05/10/2023 Labeler - Boehringer Ingelheim Animal Health USA Inc. (007134091)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.