CPDA-1- anticoagulant citrate phosphate dextrose adenine solution
CPDA-1 by
Drug Labeling and Warnings
CPDA-1 by is a Prescription medication manufactured, distributed, or labeled by Fenwal, Inc., Fenwal International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
Instrucciones para la recolección de sangre Unidad BOLSANG™ (CPDA-1 o CPD/ADSOL™)
Sólamente Rx
Antes de continuar, identifique la unidad BOLSANG™ utilizando el sistema de identificación de donante adecuado.
1. Suspenda la unidad BOLSANG tan abajo del brazo del donante como sea posible.
2. Aplique un manguito de presión sanguínea o torniquete.
3. Desinfecte el sitio de la venipunción.
4. Ate el torniquete o infle la manga de presión a 60 mm Hg.
5. Sujete la aguja y/o el tubo del donante correctamente.
6. Cierre el tubo del donante, retire el capuchón de la aguja y realice la venipunción.
7. Libere la abrazadera del tubo del donante para permitir el flujo de sangre.
8. Mezcle la sangre y el anticoagulante en varios intervalos durante la recolección e inmediatamente después de la recolección.
9. Recolecte la cantidad de sangre que figura en la etiqueta de la bolsa. Hay anticoagulante suficiente para ± 10%.
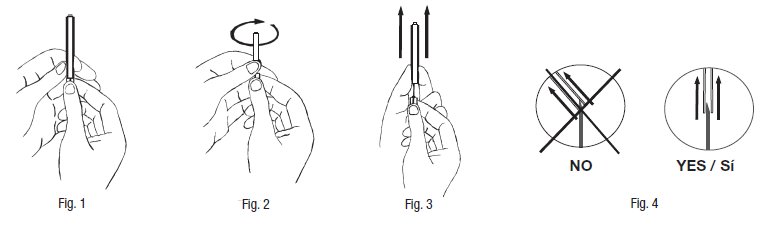
10. Selle herméticamente el tubo entre el sitio de toma de muestras y la bolsa de recolección primaria, a aprox. 3 cm del sitio de toma de muestras. (A esta altura, se puede utilizar un termosellador o gancho de metal u otro método para sellar). Exprima el tubo del donante ± 10 cm desde la abrazadera hacia la bolsa de recolección, doble el tubo y coloque un gancho de metal (4R4418) sobre la parte exprimida del tubo. Para recolectar muestras, ensamble un adaptador de aguja de llave Luer y un soporte para tubos.
11. Inserte el conjunto en sentido horario con firmeza dentro del sitio de toma de muestras, y asegúrese de que la llave Luer esté completamente dentro del sitio de toma de muestras. Tome las muestras de sangre con tubos para muestras al vacío.
12. Aísle la bolsa de sangre cortando el tubo (o rompiendo el sello) entre la pinza hemostática y el gancho de metal.
13. Retire el torniquete o libere la presión de manga y retire la aguja. Deseche el conjunto de la aguja en un recipiente adecuado para residuos de peligros biológicos siguiendo los procedimientos establecidos.
14. Si es necesario, exprima sangre del tubo del donante dentro de la unidad BOLSANG, mezcle y deje que el tubo vuelva a llenarse. Repita todos los pasos una vez. Selle en las marcas X, comenzando por el extremo sellado del tubo del donante hacia la bolsa, para proporcionar alícuotas numeradas de sangre anticoagulada para las pruebas cruzadas.
15. Retire y deseche el sitio de toma de muestras en Y y el protector de aguja DONORCARE™ en un recipiente adecuado para residuos de peligros biológicos siguiendo los procedimientos establecidos.
16. Preparación de componentes (separación manual):
El plasma fresco congelado debe separarse de los glóbulos rojos y colocarse en el congelador a -18°C o menos dentro de las 8 horas posteriores a la recolección de sangre.
Si corresponde, se debe agregar solución conservadora de glóbulos rojos ADSOL™ a los glóbulos rojos inmediatamente después de retirar el plasma. La preparación de los glóbulos rojos AS-1 puede variar según la opción de procesamiento seleccionada:
a)Dentro de las 8 horas posteriores a la recolección de sangre, si la sangre entera se conserva a temperatura ambiente.
b)Dentro de los 3 días posteriores a la recolección de sangre si la sangre entera se refrigera.
Para la preparación de componentes con extractores de plasma manuales, use técnicas estándares de procesamiento y almacenamiento.
Si debe prepararse concentrado de plaquetas, debe separarse de los glóbulos rojos dentro de las 8 horas posteriores a la recolección de sangre.
17. Al procesar una unidad BOLSANG múltiple, centrifugue los recipientes primario y secundario para preparar glóbulos rojos CPD o glóbulos rojos CPDA-1, según corresponda.
18. Coloque el recipiente primario en el extractor de plasma y exprima el plasma para colocarlo en un contenedor Bolsa de transferencia vacío liberando la placa de presión y abriendo el cierre del tubo del recipiente primario. (Rompa la cánula en la parte superior de la bolsa primaria sujetando la parte rígida y doblándola 3 veces a 90° en sentido lateral. Asegúrese de que el plasma fluya a la Bolsa de transferencia).
19. Cuando se haya retirado la cantidad de plasma deseada, cierre el tubo entre el sitio en Y y el recipiente de plasma.
20. Si corresponde, suspenda el recipiente de solución conservadora de glóbulos rojos ADSOL, abra el cierre del tubo (rompa el vástago de la cánula con la otra mano doblándolo hacia adelante y atrás, a 90°, por lo menos, 3 veces. Asegúrese de que la cánula esté bien rota colocando la parte rota en la parte superior de la carcasa) y drene el contenido dentro del recipiente primario de los glóbulos rojos CPD. Cierre el tubo.
21. Selle el tubo de transferencia en tres lugares después del segundo número de segmento, cerca del recipiente primario (dejando dos números de segmento conectados al recipiente primario) y corte el sello del medio con cuidado de no salpicar líquido. Para los códigos de unidades BOLSANG dobles, deseche el recipiente de solución ADSOL. Para otros códigos de ADSOL, el recipiente de solución vacío puede usarse como contenedor Bolsa de transferencia para preparar más componentes.
22. Si corresponde, mezcle bien la solución conservadora de glóbulos rojos ADSOL con los glóbulos rojos.
23. Preparación de componentes (OPTIPAC™/OPTIPRESS™):
Remítase al manual de OPTIPRESS para ver las instrucciones detalladas.
Para la preparación de componentes con un OPTIPAC, use técnicas estándares de procesamiento y almacenamiento.
24. Centrifugue la bolsa para separar el componente sanguíneo.
25. Coloque la bolsa primario de OPTIPAC en el OPTIPRESS.
26. Ponga el recipiente de solución aditiva en el banco y pase el tubo por la abrazadera inferior.
27. Coloque las Bolsas de transferencia vacíos sobre el OPTIPRESS y pase el tubo por la abrazadera superior.
28. Ponga en marcha el OPTIPRESS presionando el botón “Press” (Presionar).
29. Rompa la cánula en la parte superior de la bolsa primaria sujetando la parte rígida y doblándola 3 veces a 90° en sentido lateral. Asegúrese de que el plasma fluya a la Bolsa de transferencia.
30. Tan pronto como la abrazadera superior esté cerrada, sostenga la base de la cánula sobre el recipiente de solución aditiva, entre el pulgar y el índice. Rompa el vástago de la cánula con la otra mano doblándola hacia adelante y hacia atrás, a 90°, por lo menos, 3 veces. Asegúrese de que la cánula esté bien rota ubicando la parte rota en la parte superior de la carcasa.
31. Cuando haya completado el procedimiento, ambas abrazaderas, la superior y la inferior, ocluyen el tubo para impedir la contaminación con glóbulos rojos. En ese momento, coloque un gancho en la línea en la parte superior del OPTIPAC para impedir que los glóbulos rojos suban por la línea de plasma cuando se haya quitado el OPTIPAC del OPTIPRESS.
32. Selle la Bolsa de transferencia que contiene plasma y la unidad de glóbulos rojos, y deje el OPTIPAC y la bolsa de plaquetas.
33. Presione el botón “PRESS” (Presionar) del OPTIPRESS para liberar la placa de presión y quitar las bolsas.
34. Después de almacenar el OPTIPAC, la bolsa primaria y el recipiente de plaquetas vacío pueden usarse para preparar otros componentes, según los procedimientos del centro de sangre.
35. Almacenamiento:
Almacene los Glóbulos Rojos AS-1 suspendidos o la Sangre Entera/los Glóbulos Rojos CPDA-1 suspendidos (según corresponda) a una temperatura de entre 1 y 6°C.
Utilice los Glóbulos Rojos AS-1 en infusión dentro de los 42 días posteriores a la recolección y la Sangre Entera/los Glóbulos Rojos CPDA-1 dentro de los 35 días posteriores a la recolección.
NOTAS:
- Proteger la bolsa y el tubo de objetos cortantes.
- Al congelar, el plástico es más frágil.
PRECAUCIONES A TOMARSE
- No usar si hay signos visibles de deterioro.
- No usar si los protectores del paso de fluido no están en su sitio o no están intactos.
- No usar a menos que la solución tenga un aspecto transparente y no se detecten pérdidas.
Superficie interior estéril y libre de pirógenos. Esterilización por vapor. Utilice sólo una vez. No ventilar. Deseche el envase según corresponda.Almacenar a temperatura ambiente controlada.
Farmacopea de los Estados Unidos. “Avisos Generales” United States Pharmacopeial Convention, Inc., 12601 Twinbrook Parkway, Rockville, MD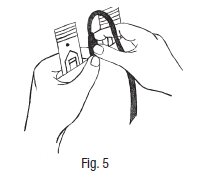
-
Instructions for Blood Collection (CPDA-1 or CPD/ADSOL™) BLOOD-PACK™ Unit
Rx only
Before proceeding further, identify BLOOD-PACK™ unit using appropriate donor identification system.
- 1. Suspend the BLOOD-PACK unit as far below donor’s arm as possible.
- 2. Apply blood pressure cuff or tourniquet.
- 3. Disinfect site of venipuncture.
- 4. Tie tourniquet or inflate pressure cuff to 60 mm Hg.
- 5. Appropriately secure donor needle and/or tubing.
- 6. Clamp donor tube, remove needle cover and perform venipuncture.
- 7. Release clamp from donor tube to permit flow of blood.
- 8. Mix blood and anticoagulant at several intervals during collection and immediately after collection.
- 9. Collect the quantity of blood stated on the pack label. Anticoagulant is sufficient for ± 10%.
- 10. Hermetically seal the tube between the sampling site and primary collection bag approx. 3 cm from sample site. (A heat sealer or metal clip or other appropriate method may be used to seal at this point). Strip the donor tubing ± 10 cm from the clamp toward the collection pack, bend the tubing and apply a metal clip (4R4418) onto the stripped portion of the tubing. To collect samples assemble a Luer needle adapter and tube holder.
- 11. Insert the assembly clockwise firmly and positively into the sample site ensuring the Luer is fully entered into the sample site. Proceed to blood sampling with evacuated sample tubes.
- 12. Isolate the BLOOD-PACK by cutting the tubing (or breaking the seal) between the hemostat and metal clip.
- 13. Remove tourniquet or release pressure in cuff and withdraw needle. Dispose of needle assembly in an appropriate biohazardous waste container following established procedures.
- 14. If necessary, strip blood from donor tube into the BLOOD-PACK unit, mix and allow tube to refill. Repeat once. Seal at X marks, beginning at sealed end of donor tube toward pack, to provide numbered aliquots of anticoagulated blood for crossmatching.
- 15. Remove and discard the Y-Sampling Site and the DONORCARE™ Needle Guard into an appropriate biohazardous waste container following established procedures.
- 16. Component Preparation (Manual separation):
- Fresh Frozen Plasma should be separated from the red blood cells and placed in the freezer at -18°C or colder within 8 hours after blood collection.
- If applicable, ADSOL™ red cell preservation solution should be added to the red blood cells immediately after the removal of plasma. Preparation of AS-1 red blood cells may vary depending on processing option selected:
- a) Within 8 hours of blood collection if whole blood is held at ambient temperature.
- b) Within 3 days of blood collection if whole blood is refrigerated.
- For component preparation using manual plasma extractors, use standard component processing and storage techniques.
- If a platelet concentrate is to be prepared, it should be separated from the red blood cells within 8 hours after blood collection.
- 17. When processing a multiple BLOOD-PACK unit, centrifuge primary and secondary containers to prepare CPD red blood cells, or CPDA-1 red blood cells as applicable.
- 18. Place primary container in plasma extractor and express plasma into empty transfer pack container by releasing pressure plate and opening closure in tubing of primary container. (Break the cannula on top of the primary pack by holding the rigid part and bending 3 times laterally 90°. Make sure the plasma flows to the transfer pack).
- 19. When desired amount of plasma has been removed, clamp tubing between Y and plasma container.
- 20. If applicable, suspend ADSOL red cell preservation solution container, open closure in tubing (Break the stem of the cannula using the other hand by bending back and forth at 90° at least 3 times. Ensure the cannula is well broken by locating the broken part in the upper part of the housing) and drain contents into primary container of CPD red blood cells. Clamp tubing.
- 21. Seal transfer tubing in three places after second segment number near primary container (leaving two segment numbers connected to the primary container) and cut middle seal being careful to avoid fluid splatter. For Double BLOOD-PACK unit codes, discard ADSOL solution container. For other ADSOL codes, the empty solution container may be used as a transfer pack container for further component preparation.
- 22. If applicable, mix ADSOL red cell preservation solution and red cells thoroughly.
- 23. Component Preparation (OPTIPAC™/OPTIPRESS™):
- Refer to OPTIPRESS manual for detailed instructions.
- For component preparation using an OPTIPAC, use standard component processing and storage techniques.
- 24. Centrifuge the pack to separate blood component.
- 25. Position the OPTIPAC primary pack in the OPTIPRESS.
- 26. Put the additive solution container on the bench and the tubing through the lower clamp.
- 27. Put the empty transfer packs on top of the OPTIPRESS and tubing through the upper clamp.
- 28. Start the OPTIPRESS by pushing the “Press” button.
- 29. Break the cannula on top of the primary pack by holding the rigid part and bending 3 times laterally 90°. Make sure the plasma flows to the transfer pack.
- 30. As soon as the top clamp is closed, hold the base of the cannula on the additive solution container between the thumb and first finger. Break the stem of the cannula using the other hand by bending back and forth at 90° at least 3 times. Ensure the cannula is well broken by locating the broken part in the upper part of the housing.
- 31. When the procedure is completed, both the upper and lower clamps occlude the tubing so as to prevent red cell contamination. At this point place a clip on the line at the top of the OPTIPAC to prevent any red cells travelling up the plasma line when the OPTIPAC has been removed from the OPTIPRESS.
- 32. Seal the transfer pack containing plasma and the unit of red cells, leaving behind the OPTIPAC and the platelet pack.
- 33. Push the “PRESS” button on the OPTIPRESS to release the pressure plate and remove the packs.
- 34. After storage of the OPTIPAC, the primary pack, and the empty platelet container may be used to prepare other components according to blood center procedures.
- 35. Storage:
- Store suspended AS-1 red blood cells or suspended CPDA-1 whole blood/red blood cells (as applicable) between 1 and 6°C.
- Infuse AS-1 red blood cells within 42 days of collection, and CPDA-1 whole blood/red blood cells within 35 days of collection.
NOTES:
- - Protect container and tubing from sharp objects.
- - When frozen, plastic is more fragile.
CAUTIONS TO BE TAKEN
- - Do not use if there is visible sign of deterioration.
- - Do not use if fluid path closures are loose or not intact.
- - Do not use unless solution is clear and no leaks are detected.
Sterile, non-pyrogenic fluid path. Steam sterilized.
Single use only. Do not vent.
Dispose of container appropriately.
Store at Controlled Room Temperature. USP Definition of “Controlled Room Temperature” United States Pharmacopeia, General Notices.
United States Pharmacopeial Convention, Inc.
12601 Twinbrook Parkway, Rockville, MD – Fabricante/Manufacturer
– Fabricante/Manufacturer Fabricado por/Manufactured by:
Fabricado por/Manufactured by:
Fenwal International, Inc.
Road 357, Km. 0.8
Maricao, PR 00606Hecho en EE. UU. / Made in USA
Importado y distribuido en India por:/
Imported and distributed in India by:
Fenwal India Pvt LtdUpper Ground Floor, Tower B
DLF Building No. 10, DLF Cyber City
DLF Phase II, Gurgaon 122 002,
Haryana, India
Import License No.: FF-504-14890Importado y distribuido en Indonesia por:/
Imported and distributed in Indonesia by:
PT. Medquest Jaya Global
Menara Salemba 6th Floor
Jl.Salemba Raya Kav 5-5A
Jakarta-Indonesia 10440Importado y distribuido en Thailand por:/
Imported and distributed in Thailand by:
Fenwal (Thailand) Ltd.
17th Fl. Thanapoom Tower
1550 New Petchburi Rd., Makasan
Rajthevi, Bangkok 10400
Thailand
Reg. No.:Importado y distribuido en Perú por:/
Imported and distributed in Perú by:
HERSIL S.A. LABORATORIOS INDUSTRIALES FARMACÉUTICOS
Av. Los Frutales No. 220
Ate, Lima 3 –Perú
RUC: 20100060150
Q.F. Responsable: Antonio Benitez Z.
Reg. No.:Importado y distribuido en Venezuela por:/
Imported and distributed in Venezuela by:
SOLUCARE GT, C.A. RIF J-30512494-9
en La Urbina, Caracas,
Republica Bolivariana de Venezuela
TELF (0212) 2436663
Representante legal: Ynecel C. Obando M.MSAS 5635Importado y distribuido en Colombia por:/
Imported and distributed in Colombia by:
Fenwal Colombia Ltda.
Centroempresa, Cali, Colombia
Reg. No.: INVIMA-2006M-0000140-R107-19-04-282 REV: A 01/2010
FENWAL, BOLSANG, ADSOL, OPTIPRESS y OPTIPAC son Marcas Registradas de Fenwal, Inc.
FENWAL, BLOOD-PACK, ADSOL, OPTIPRESS and OPTIPAC are trademarks of Fenwal, Inc.
DONORCARE is a trademark of ITL Corporation.
DONORCARE es una marca comercial de ITL Corporación.
© 2010 Fenwal, Inc. Todos los derechos reservados. All rights reserved.
-
PACKAGE/LABEL DISPLAY PANEL
REF R4R7295
8 Units
Fenwal™
Double Blood-Pack™ Unit Anticoagulant Citrate Phosphate Dextrose Adenine Solution (CPDA-1)
PL 146 Plastic
Rx Only
Double Blood-Pack unit consisting of a primary pack containing 63 mL of CPDA-1 anticoagulant solution and one transfer pack without solution for collection of 450 mL of blood, 16 gauge needle.
See instructions for use. Sterile, non-pyrogenic fluid path. Steam sterilized. Single use only. Do not vent. Do not use if there is any visible sign of deterioration. Dispose of container appropriately. Store at Controlled Room Temperature (refer to direction insert).
Unused packs in open foil pouch may be kept 60 days by folding and securing open end of foil pouch, to prevent possible loss of moisture.
Direct handling of product surfaces prior to extended storage in the foil pack may result in mold growth.
Units removed from the foil pouch must be used within 4 days (96 hours). Units out of the foil pouch for longer than 4 days must be discarded.
CPDA-1 formula: Each unit consists of a primary container with 63 mL of CPDA-1 USP Solution. Each 63 mL CPDA-1 solution contains 2.01 g Dextrose (monohydrate), USP, 1.66 g Sodium Citrate (dihydrate), USP, 206 mg Citric Acid (monohydrate), USP, 140 mg Monobasic Sodium Phosphate (monohydrate), USP, 17.3 mg Adenine, USP. Water for Injection USP to 63 mL.
Manufactured by:
Fenwal International, Inc.
Road 357, Km. 0.8
Maricao, PR 00606Made in USA
Imported and distributed in India by:
Fenwal India Pvt Ltd
Upper Ground Floor, Tower B
DLF Building No. 10
DLF Cyber City, DLF Phase II
Gurgaon 122 002,
Haryana, India
Import License No.: FF-504-14890
MRP Rs: 169 (Inclusive of all taxes)Imported and distributed in Indonesia by:
PT. Medquest Jaya Global
Menara Salemba 6th Floor
Jl.Salemba Raya Kav 5-5A
Jakarta-Indonesia 10440
Reg. No.: See instructions for use.FENWAL and BLOOD-PACK are trademarks of Fenwal, Inc.
07-28-03-910 REV: A

-
INGREDIENTS AND APPEARANCE
CPDA-1
anticoagulant citrate phosphate dextrose adenine solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0942-6330 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Dextrose Monohydrate (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) Dextrose Monohydrate 2 g in 63 mL Trisodium Citrate Dihydrate (UNII: B22547B95K) (Anhydrous Citric Acid - UNII:XF417D3PSL) Anhydrous Citric Acid 1.66 g in 63 mL Anhydrous Citric Acid (UNII: XF417D3PSL) (Anhydrous Citric Acid - UNII:XF417D3PSL) Anhydrous Citric Acid 188 mg in 63 mL Sodium Phosphate, Monobasic, Monohydrate (UNII: 593YOG76RN) (PHOSPHATE ION - UNII:NK08V8K8HR) Sodium Phosphate, Monobasic, Monohydrate 140 mg in 63 mL Adenine (UNII: JAC85A2161) (Adenine - UNII:JAC85A2161) Adenine 17.3 mg in 63 mL Inactive Ingredients Ingredient Name Strength Sodium Hydroxide (UNII: 55X04QC32I) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0942-6330-02 63 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA BN770420 05/25/2010 Labeler - Fenwal, Inc. (794519020) Establishment Name Address ID/FEI Business Operations Fenwal International, Inc. 091164590 MANUFACTURE(0942-6330)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.