NEOMYCIN AND POLYMYXIN B SULFATES, AND BACITRACIN ZINC- neomycin sulfate, polymyxin b sulfate and bacitracin zinc ointment
Neomycin and Polymyxin B Sulfates, and Bacitracin Zinc by
Drug Labeling and Warnings
Neomycin and Polymyxin B Sulfates, and Bacitracin Zinc by is a Animal medication manufactured, distributed, or labeled by VetOne, Akorn, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- DESCRIPTION:
-
ACTIONS:
Polymyxin B is one of a group of closely related substances produced by various strains of Bacillus polymyxa. The activity of polymyxin B is sharply restricted to gram-negative bacteria. Neomycin, isolated from Streptomyces fradiae, has antibacterial activity in vitro against a wide range of gram-negative and gram-positive organisms. Bacitracin, an antibiotic substance derived from cultures of Bacillus subtilis (Tracy), exerts antibacterial action in vitro against a variety of gram-positive and a few gram-negative organisms.
-
INDICATIONS:
This product is indicated for the treatment of superficial bacterial infections of the eyelid and conjunctiva of dogs and cats when due to organisms susceptible to one or more of the antibiotics contained in the ointment.
Laboratory tests should be conducted including in vitro culturing and susceptibility tests on samples collected prior to treatment.
-
PRECAUTIONS:
If irritation develops discontinue treatment with this drug. If there is no response to treatment in 2 to 3 days, discontinue treatment and re-evaluate diagnosis. Prolonged use may result in overgrowth of nonsusceptible organisms, including fungi.
Care should be taken not to contaminate the applicator tip during administration of the preparation.
- ADVERSE REACTIONS:
-
DOSAGE AND ADMINISTRATION:
Properly cleanse area to be treated. Foreign bodies, crusted exudates and debris should be carefully removed. Express a small quantity of ointment into the conjunctival sac beneath the lower eyelid three or four times daily. After application hold the eyelids shut for a short time so that a thin film of ointment covers the cornea.
- HOW SUPPLIED:
-
STORAGE:
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temp].
KEEP OUT OF REACH OF CHILDREN.
Not for Human Use.
CAUTION: Federal (U.S.A.) law restricts this drug to use by or on the order of a licensed veterinarian.
Warning: Serious hypersensitivity (anaphylactic) reactions have been reported in cats within 4 hours of application of antibiotic ophthalmic preparations. Some of these reactions have resulted in death.
VET one®
Distributed by: MWI
Boise, ID 83705
www.VetOne.net
MWNB00n Rev. 03/18
- PRINCIPAL DISPLAY PANEL
-
PRINCIPAL DISPLAY PANEL
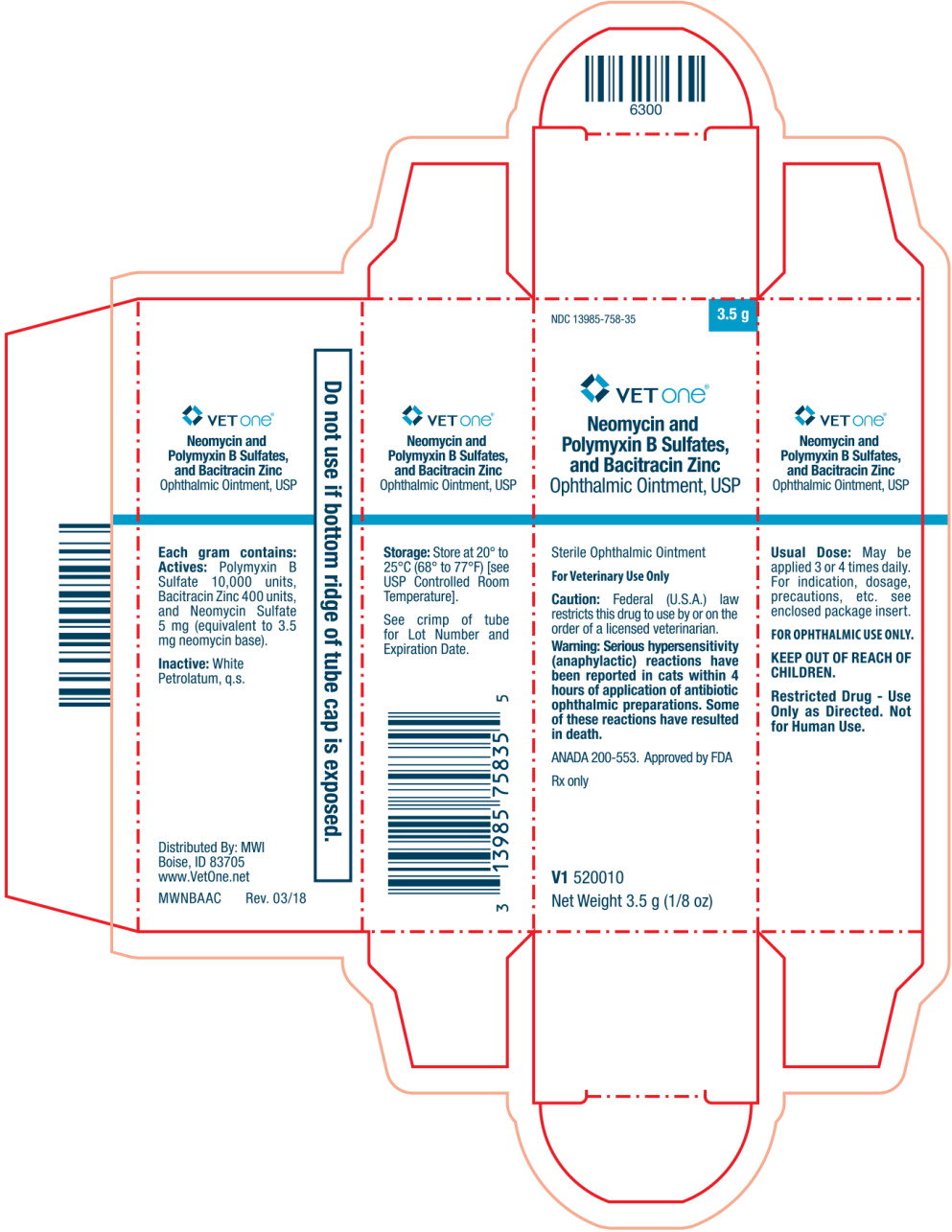
Principal Display Panel Text for Carton Label:
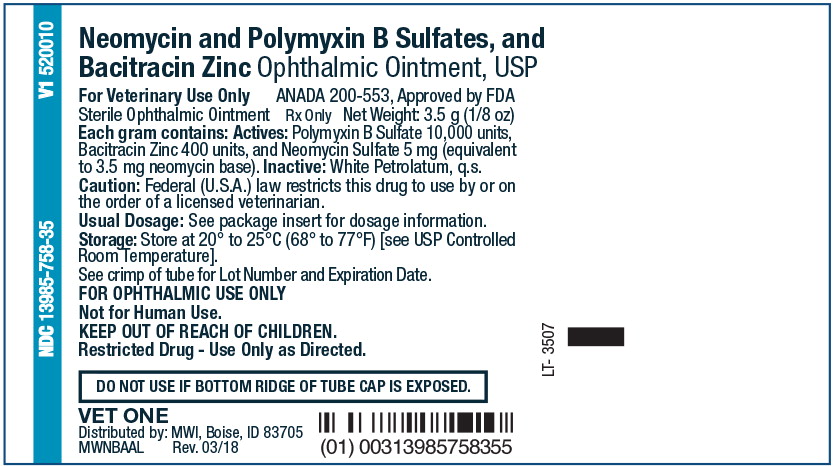
NDC: 13985-758-35 3.5 g
VET One® Logo
Neomycin and
Polymyxin B Sulfates,
and Bacitracin Zinc
Ophthalmic Ointment, USP
Sterile Ophthalmic Ointment
For Veterinary Use Only
Caution: Federal (U.S.A.) law
restricts this drug to use by or on the
order of licensed veterinarian.
Warning: Serious hypersensitivity
(anaphylactic) reactions have
been reported in cats within 4
hours of application of antibiotic
ophthalmic preparations. Some
of these reactions have resulted
in death.
ANADA 200-553. Approved by FDA
Rx only
V1 520010
Net Weight: 3.5 g (1/8 oz)
-
INGREDIENTS AND APPEARANCE
NEOMYCIN AND POLYMYXIN B SULFATES, AND BACITRACIN ZINC
neomycin sulfate, polymyxin b sulfate and bacitracin zinc ointmentProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 13985-758 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Neomycin Sulfate (UNII: 057Y626693) (Neomycin - UNII:I16QD7X297) Neomycin 3.5 mg in 1 g Polymyxin B Sulfate (UNII: 19371312D4) (Polymyxin B - UNII:J2VZ07J96K) Polymyxin B 10000 [USP'U] in 1 g Bacitracin Zinc (UNII: 89Y4M234ES) (Bacitracin - UNII:58H6RWO52I) Bacitracin 400 [USP'U] in 1 g Inactive Ingredients Ingredient Name Strength Petrolatum (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13985-758-35 1 in 1 CARTON 1 3.5 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200553 06/14/2018 Labeler - VetOne (019926120) Registrant - Akorn, Inc. (062649876) Establishment Name Address ID/FEI Business Operations Akorn, Inc. 603980319 MANUFACTURE, ANALYSIS, STERILIZE, LABEL, PACK
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.