LYSTEDA- tranexamic acid tablet
Lysteda by
Drug Labeling and Warnings
Lysteda by is a Prescription medication manufactured, distributed, or labeled by Ferring Pharmaceuticals Inc., Mikart, LLC, Kyowa Pharma Chemical Co. Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use LYSTEDA safely and effectively. See full prescribing information for LYSTEDA.
LYSTEDA® (tranexamic acid, USP) Tablets
Initial U.S. Approval: 1986INDICATIONS AND USAGE
LYSTEDA (tranexamic acid, USP) Tablets is an antifibrinolytic indicated for the treatment of cyclic heavy menstrual bleeding. (1)
DOSAGE AND ADMINISTRATION
- 1,300 mg (two 650 mg tablets) three times a day (3,900 mg/day) for a maximum of 5 days during monthly menstruation (2.1)
- Renal impairment: Dosage adjustment is needed if serum creatinine concentration (Cr) is higher than 1.4 mg/dL (2.2)
- Cr above 1.4 mg/dL and ≤ 2.8 mg/dL: 1,300 mg (two 650 mg tablets) two times a day (2,600 mg/day) for a maximum of 5 days during menstruation
- Cr above 2.8 mg/dL and ≤ 5.7 mg/dL: 1,300 mg (two 650 mg tablets) once a day (1,300 mg/day) for a maximum of 5 days during menstruation
- Cr above 5.7 mg/dL: 650 mg (one 650 mg tablet) once a day (650 mg/day) for a maximum of 5 days during menstruation
DOSAGE FORMS AND STRENGTHS
Tablets: 650 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Concomitant use of LYSTEDA with Factor IX complex concentrates, anti-inhibitor coagulant concentrates or all-trans retinoic acid (oral tretinoin) may increase the risk of thrombosis. (5.1)
- Visual or ocular adverse effects may occur with LYSTEDA. Immediately discontinue use if visual or ocular symptoms occur. (5.1)
- In case of severe allergic reaction, discontinue LYSTEDA and seek immediate medical attention. (5.2)
- Cerebral edema and cerebral infarction may be caused by use of LYSTEDA in women with subarachnoid hemorrhage. (5.3)
- Ligneous conjunctivitis has been reported in patients taking tranexamic acid. (5.4)
ADVERSE REACTIONS
Most common adverse reactions in clinical trials (≥ 5%, and more frequent in LYSTEDA subjects compared to placebo subjects) are headache, sinus and nasal symptoms, back pain, abdominal pain, musculoskeletal pain, joint pain, muscle cramps, migraine, anemia and fatigue. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Ferring Pharmaceuticals Inc. at 1-888-FERRING (1-888-337-7464) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Concomitant therapy with tissue plasminogen activators may decrease the efficacy of both LYSTEDA and tissue plasminogen activators. (7.2)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 4/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Renal Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Thromboembolic Risk
4.2 Hypersensitivity to Tranexamic Acid
5 WARNINGS AND PRECAUTIONS
5.1 Thromboembolic Risk
5.2 Severe Allergic Reaction
5.3 Subarachnoid Hemorrhage
5.4 Ligneous Conjunctivitis
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Hormonal Contraceptives
7.2 Tissue Plasminogen Activators
7.3 Factor IX Complex Concentrates or Anti-Inhibitor Coagulant Concentrates
7.4 All-Trans Retinoic Acid (Oral Tretinoin)
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy (Category B)
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Three-Cycle Treatment Study
14.2 Six-Cycle Treatment Study
14.3 MBL Results over Time
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
LYSTEDA® (tranexamic acid, USP) Tablets is indicated for the treatment of cyclic heavy menstrual bleeding [see Clinical Studies (14)].
Prior to prescribing LYSTEDA, exclude endometrial pathology that can be associated with heavy menstrual bleeding.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dose of LYSTEDA for women with normal renal function is two 650 mg tablets taken three times daily (3900 mg/day) for a maximum of 5 days during monthly menstruation. LYSTEDA may be administered without regard to meals. Tablets should be swallowed whole and not chewed or broken apart.
2.2 Renal Impairment
In patients with renal impairment, the plasma concentration of tranexamic acid increased as serum creatinine concentration increased [see Clinical Pharmacology (12.3)]. Dosage adjustment is needed in patients with serum creatinine concentration higher than 1.4 mg/dL (Table 1).
Table 1. Dosage of LYSTEDA in Patients with Renal Impairment LYSTEDA Serum Creatinine (mg/dL) Adjusted Dose Total Daily Dose Cr above 1.4 and ≤ 2.8 1300 mg (two 650 mg tablets) two times a day for a maximum of 5 days during menstruation 2600 mg Cr above 2.8 and ≤ 5.7 1300 mg (two 650 mg tablets) once a day for a maximum of 5 days during menstruation 1300 mg Cr above 5.7 650 mg (one 650 mg tablet) once a day for a maximum of 5 days during menstruation 650 mg - 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
4.1 Thromboembolic Risk
Do not prescribe LYSTEDA to women who are
- using combination hormonal contraception
- known to have any of the following conditions:
- Active thromboembolic disease (e.g., deep vein thrombosis, pulmonary embolism, or cerebral thrombosis)
- A history of thrombosis or thromboembolism, including retinal vein or artery occlusion
- An intrinsic risk of thrombosis or thromboembolism (e.g., thrombogenic valvular disease, thrombogenic cardiac rhythm disease, or hypercoagulopathy)
Venous and arterial thrombosis or thromboembolism, as well as cases of retinal artery and retinal vein occlusions, have been reported with tranexamic acid.
4.2 Hypersensitivity to Tranexamic Acid
Do not prescribe LYSTEDA to women with known hypersensitivity to tranexamic acid [see Warnings and Precautions (5.2) and Adverse Reactions (6.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Thromboembolic Risk
Concomitant Use of Hormonal Contraceptives
Combination hormonal contraceptives are known to increase the risk of venous thromboembolism, as well as arterial thromboses such as stroke and myocardial infarction. Because LYSTEDA is antifibrinolytic, the risk of venous thromboembolism, as well as arterial thromboses such as stroke, may increase further when hormonal contraceptives are administered with LYSTEDA. This is of particular concern in women who are obese or smoke cigarettes, especially smokers over 35 years of age.
Women using hormonal contraception were excluded from the clinical trials supporting the safety and efficacy of LYSTEDA, and there are no clinical trial data on the risk of thrombotic events with the concomitant use of LYSTEDA with hormonal contraceptives. However, there have been US postmarketing reports of venous and arterial thrombotic events in women who have used LYSTEDA concomitantly with combination hormonal contraceptives. For this reason, concomitant use of LYSTEDA with combination hormonal contraceptives is contraindicated. [see Contraindications (4.1) and Drug Interactions (7.1)].
Factor IX Complex Concentrates or Anti-Inhibitor Coagulant Concentrates
LYSTEDA is not recommended for women taking either Factor IX complex concentrates or anti-inhibitor coagulant concentrates because the risk of thrombosis may be increased [see Drug Interactions (7.3) and Clinical Pharmacology (12.3)].
All-Trans Retinoic Acid (Oral Tretinoin)
Exercise caution when prescribing LYSTEDA to women with acute promyelocytic leukemia taking all-trans retinoic acid for remission induction because of possible exacerbation of the procoagulant effect of all-trans retinoic acid [see Drug Interactions (7.4) and Clinical Pharmacology (12.3)].
Ocular Effects
Retinal venous and arterial occlusion has been reported in patients using tranexamic acid. Patients should be instructed to report visual and ocular symptoms promptly. In the event of such symptoms, patients should be instructed to discontinue LYSTEDA immediately and should be referred to an ophthalmologist for a complete ophthalmic evaluation, including dilated retinal examination, to exclude the possibility of retinal venous or arterial occlusion.
5.2 Severe Allergic Reaction
A case of severe allergic reaction to LYSTEDA was reported in the clinical trials, involving a subject who experienced dyspnea, tightening of her throat, and facial flushing that required emergency medical treatment. A case of anaphylactic shock has also been reported in the literature, involving a patient who received an intravenous bolus of tranexamic acid.
-
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Short-term Studies
The safety of LYSTEDA in the treatment of heavy menstrual bleeding (HMB) was studied in two randomized, double-blind, placebo-controlled studies [see Clinical Studies (14)]. One study compared the effects of two doses of LYSTEDA (1950 mg and 3900 mg given daily for up to 5 days during each menstrual period) versus placebo over a 3-cycle treatment duration. A total of 304 women were randomized to this study, with 115 receiving at least one dose of 3900 mg/day of LYSTEDA. A second study compared the effects of LYSTEDA (3900 mg/day) versus placebo over a 6-cycle treatment duration. A total of 196 women were randomized to this study, with 117 receiving at least one dose of LYSTEDA. In both studies, subjects were generally healthy women who had menstrual blood loss of ≥ 80 mL.
In these studies, subjects were 18 to 49 years of age with a mean age of approximately 40 years, had cyclic menses every 21-35 days, and a BMI of approximately 32 kg/m2. On average, subjects had a history of HMB for approximately 10 years and 40% had fibroids as determined by transvaginal ultrasound. Approximately 70% were Caucasian, 25% were Black, and 5% were Asian, Native American, Pacific Islander, or Other. Seven percent (7%) of all subjects were of Hispanic origin. Women using hormonal contraception were excluded from the trials.
The rates of discontinuation due to adverse events during the two clinical trials were comparable between LYSTEDA and placebo. In the 3-cycle study, the rate in the 3900 mg LYSTEDA dose group was 0.8% as compared to 1.4% in the placebo group. In the 6-cycle study, the rate in the LYSTEDA group was 2.4% as compared to 4.1% in the placebo group. Across the studies, the combined exposure to 3900 mg/day LYSTEDA was 947 cycles and the average duration of use was 3.4 days per cycle.
A list of adverse events occurring in ≥ 5% of subjects and more frequently in LYSTEDA treated subjects receiving 3900 mg/day compared to placebo is provided in Table 2.
Table 2. Adverse Events Reported by ≥ 5% of Subjects Treated with LYSTEDA and More Frequently in LYSTEDA-treated Subjects LYSTEDA
3900 mg/day
n (%)
(N=232)Placebo
n (%)
(N=139)- * Includes headache and tension headache
- † Nasal and sinus symptoms include nasal, respiratory tract and sinus congestion, sinusitis, acute sinusitis, sinus headache, allergic sinusitis and sinus pain, and multiple allergies and seasonal allergies
- ‡ Abdominal pain includes abdominal tenderness and discomfort
- § Musculoskeletal pain includes musculoskeletal discomfort and myalgia
- ¶ Arthralgia includes joint stiffness and swelling
Total Number of Adverse Events 1500 923 Number of Subjects with at Least One Adverse Event 208 (89.7%) 122 (87.8%) HEADACHE * 117 (50.4%) 65 (46.8%) NASAL & SINUS SYMPTOMS † 59 (25.4%) 24 (17.3%) BACK PAIN 48 (20.7%) 21 (15.1%) ABDOMINAL PAIN ‡ 46 (19.8%) 25 (18.0%) MUSCULOSKELETAL PAIN § 26 (11.2%) 4 (2.9%) ARTHRALGIA ¶ 16 (6.9%) 7 (5.0%) MUSCLE CRAMPS & SPASMS 15 (6.5%) 8 (5.8%) MIGRAINE 14 (6.0%) 8 (5.8%) ANEMIA 13 (5.6%) 5 (3.6%) FATIGUE 12 (5.2%) 6 (4.3%) Long-term Studies
Long-term safety of LYSTEDA was studied in two open-label studies. In one study, subjects with physician-diagnosed heavy menstrual bleeding (not using the alkaline hematin methodology) were treated with 3900 mg/day for up to 5 days during each menstrual period for up to 27 menstrual cycles. A total of 781 subjects were enrolled and 239 completed the study through 27 menstrual cycles. A total of 12.4% of the subjects withdrew due to adverse events. Women using hormonal contraception were excluded from the study. The total exposure in this study to 3900 mg/day LYSTEDA was 10,213 cycles. The average duration of LYSTEDA use was 2.9 days per cycle.
A long-term open-label extension study of subjects from the two short-term efficacy studies was also conducted in which subjects were treated with 3900 mg/day for up to 5 days during each menstrual period for up to 9 menstrual cycles. A total of 288 subjects were enrolled and 196 subjects completed the study through 9 menstrual cycles. A total of 2.1% of the subjects withdrew due to adverse events. The total exposure to 3900 mg/day LYSTEDA in this study was 1,956 cycles. The average duration of LYSTEDA use was 3.5 days per cycle.
The types and severity of adverse events in these two long-term open-label trials were similar to those observed in the double-blind, placebo-controlled studies although the percentage of subjects reporting them was greater in the 27-month study, most likely because of the longer study duration.
A case of severe allergic reaction to LYSTEDA was reported in the extension trial, involving a subject on her fourth cycle of treatment, who experienced dyspnea, tightening of her throat, and facial flushing that required emergency medical treatment.
6.2 Postmarketing Experience
The following adverse reactions have been identified from postmarketing experience with tranexamic acid. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Based on US and worldwide postmarketing reports, the following have been reported in patients receiving tranexamic acid for various indications:
- Nausea, vomiting, and diarrhea
- Allergic skin reactions
- Anaphylactic shock and anaphylactoid reactions
- Thromboembolic events (e.g., deep vein thrombosis, pulmonary embolism, cerebral thrombosis, acute renal cortical necrosis, and central retinal artery and vein obstruction); cases have been associated with concomitant use of combination hormonal contraceptives
- Impaired color vision and other visual disturbances
- Dizziness
-
7 DRUG INTERACTIONS
No drug-drug interaction studies were conducted with LYSTEDA.
7.1 Hormonal Contraceptives
Because LYSTEDA is antifibrinolytic, concomitant use of hormonal contraception and LYSTEDA may further exacerbate the increased thrombotic risk associated with combination hormonal contraceptives. For this reason, concomitant use of LYSTEDA with combination hormonal contraceptives is contraindicated [see Contraindications (4) and Warnings and Precautions (5.1)].
7.2 Tissue Plasminogen Activators
Concomitant therapy with tissue plasminogen activators may decrease the efficacy of both LYSTEDA and tissue plasminogen activators. Therefore, exercise caution if a woman taking LYSTEDA therapy requires tissue plasminogen activators.
7.3 Factor IX Complex Concentrates or Anti-Inhibitor Coagulant Concentrates
LYSTEDA is not recommended for women taking either Factor IX complex concentrates or anti-inhibitor coagulant concentrates because the risk of thrombosis may be increased [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
7.4 All-Trans Retinoic Acid (Oral Tretinoin)
Exercise caution when prescribing LYSTEDA to women with acute promyelocytic leukemia taking all-trans retinoic acid for remission induction because of possible exacerbation of the procoagulant effect of all-trans retinoic acid [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy (Category B)
LYSTEDA is not indicated for use in pregnant women. Reproduction studies have been performed in mice, rats and rabbits and have revealed no evidence of impaired fertility or harm to the fetus due to tranexamic acid. However, tranexamic acid is known to cross the placenta and appears in cord blood at concentrations approximately equal to the maternal concentration. There are no adequate and well-controlled studies in pregnant women [see Nonclinical Toxicology (13.1)].
An embryo-fetal developmental toxicity study in rats and a perinatal developmental toxicity study in rats were conducted using tranexamic acid. No adverse effects were observed in either study at doses up to 4 times the recommended human oral dose of 3900 mg/day based on mg/m2 (actual animal dose 1500 mg/kg/day).
8.3 Nursing Mothers
Tranexamic acid is present in the mother's milk at a concentration of about one hundredth of the corresponding serum concentration. LYSTEDA should be used during lactation only if clearly needed.
8.4 Pediatric Use
LYSTEDA is indicated for women of reproductive age and is not intended for use in premenarcheal girls.
Based on a pharmacokinetic study in 20 adolescent females, 12 to 16 years of age, no dose adjustment is needed in the adolescent population [see Clinical Pharmacology (12.3)].
8.5 Geriatric Use
LYSTEDA is indicated for women of reproductive age and is not intended for use by postmenopausal women.
8.6 Renal Impairment
The effect of renal impairment on the pharmacokinetics of LYSTEDA has not been studied. Because tranexamic acid is primarily eliminated via the kidneys by glomerular filtration with more than 95% excreted as unchanged in urine, dosage adjustment in patient with renal impairment is needed [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
The effect of hepatic impairment on the pharmacokinetics of LYSTEDA has not been studied. Because only a small fraction of the drug is metabolized, dosage adjustment in patients with hepatic impairment is not needed [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
There are no known cases of intentional overdose with LYSTEDA and no subjects in the clinical program took more than 2 times the prescribed amount of LYSTEDA in a 24-hour period (>7800 mg/day). However, cases of overdose of tranexamic acid have been reported. Based on these reports, symptoms of overdose may include gastrointestinal (nausea, vomiting, diarrhea); hypotensive (e.g., orthostatic symptoms); thromboembolic (arterial, venous, embolic); visual impairment; mental status changes; myoclonus; or rash. No specific information is available on the treatment of overdose with LYSTEDA. In the event of overdose, employ the usual supportive measures (e.g., clinical monitoring and supportive therapy) as dictated by the patient's clinical status.
-
11 DESCRIPTION
LYSTEDA is an antifibrinolytic drug. The chemical name is trans-4-aminomethyl-cyclohexanecarboxylic acid. The structural formula is:
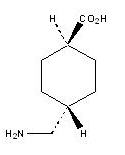
Tranexamic acid is a white crystalline powder. It is freely soluble in water and in glacial acetic acid and is very slightly soluble in ethanol and practically insoluble in ether. The molecular formula is C8H15N02 and the molecular weight is 157.2.
Tranexamic acid tablets are provided as white oval-shaped tablets and are not scored. Each tablet is debossed with the marking "FP650." The active ingredient in each tablet is 650 mg tranexamic acid. The inactive ingredients contained in each tablet are: microcrystalline cellulose, colloidal silicon dioxide, pregelatinized corn starch, povidone, hypromellose, stearic acid, and magnesium stearate.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Tranexamic acid is a synthetic lysine amino acid derivative, which diminishes the dissolution of hemostatic fibrin by plasmin. In the presence of tranexamic acid, the lysine receptor binding sites of plasmin for fibrin are occupied, preventing binding to fibrin monomers, thus preserving and stabilizing fibrin's matrix structure.
The antifibrinolytic effects of tranexamic acid are mediated by reversible interactions at multiple binding sites within plasminogen. Native human plasminogen contains 4 to 5 lysine binding sites with low affinity for tranexamic acid (Kd = 750 μmol/L) and 1 with high affinity (Kd = 1.1 μmol/L). The high affinity lysine site of plasminogen is involved in its binding to fibrin. Saturation of the high affinity binding site with tranexamic acid displaces plasminogen from the surface of fibrin. Although plasmin may be formed by conformational changes in plasminogen, binding to and dissolution of the fibrin matrix is inhibited.
12.2 Pharmacodynamics
Tranexamic acid, at in vitro concentrations of 25 - 100 µM, reduces by 20 - 60% the maximal rate of plasmin lysis of fibrin catalyzed by tissue plasminogen activator (tPA).
Elevated concentrations of endometrial, uterine, and menstrual blood tPA are observed in women with heavy menstrual bleeding (HMB) compared to women with normal menstrual blood loss. The effect of tranexamic acid on lowering endometrial tPA activity and menstrual fluid fibrinolysis is observed in women with HMB receiving tranexamic acid total oral doses of 2-3 g/day for 5 days.
In healthy subjects, tranexamic acid at blood concentrations less than 10 mg/mL has no effect on the platelet count, the coagulation time or various coagulation factors in whole blood or citrated blood. Tranexamic acid, however, at blood concentrations of 1 and 10 mg/mL prolongs the thrombin time.
Cardiac Electrophysiology
The effect of LYSTEDA on QT interval was evaluated in a randomized, single-dose, 4-way crossover study in 48 healthy females aged 18 to 49 years. Subjects received (1) LYSTEDA 1300 mg (two 650 mg tablets), (2) LYSTEDA 3900 mg (six 650 mg tablets; three times the recommended single dose), (3) moxifloxacin 400 mg, and (4) placebo. There was no significant increase in the corrected QT interval at any time up to 24 hours after the administration of either dose of LYSTEDA. Moxifloxacin, the active control, was associated with a maximum 14.11 msec mean increase in corrected QT interval (moxifloxacin – placebo) at 3 hours after administration.
12.3 Pharmacokinetics
Absorption
After a single oral administration of two 650 mg tablets of LYSTEDA, the peak plasma concentration (Cmax) occurred at approximately 3 hours (Tmax). The absolute bioavailability of LYSTEDA in women aged 18-49 is approximately 45%. Following multiple oral doses (two 650 mg tablets three times daily) administration of LYSTEDA for 5 days, the mean Cmax increased by approximately 19% and the mean area under the plasma concentration-time curve (AUC) remained unchanged, compared to a single oral dose administration (two 650 mg tablets). Plasma concentrations reached steady state at the 5th dose of LYSTEDA on Day 2.
The mean plasma pharmacokinetic parameters of tranexamic acid determined in 19 healthy women following a single (two 650 mg tablets) and multiple (two 650 mg tablets three times daily for 5 days) oral dose of LYSTEDA are shown in Table 3.
Table 3. Mean (CV%) Pharmacokinetic Parameters Following a Single (two 650 mg tablets) and Multiple Oral Dose (two 650 mg tablets three time daily for 5 days) Administration of LYSTEDA in 19 Healthy Women under Fasting Conditions Parameter Arithmetic Mean (CV%) Single dose Multiple dose Cmax = maximum concentration AUCtldc = area under the drug concentration curve from time 0 to time of last determinable concentration AUCinf = area under the drug concentration curve from time 0 to infinity Tmax = time to maximum concentration t1/2 = terminal elimination half-life - * AUC 0-tau (mcg∙h/mL) = area under the drug concentration curve from time 0 to 8 hours
- † Data presented as median (range)
Cmax (mcg/mL) 13.83 (32.14) 16.41 (26.19) AUCtldc (mcg∙h/mL) 77.96 (31.14) 77.67 * (29.39) AUCinf (mcg∙h/mL) 80.19 (30.43) - Tmax (h)† 2.5 (1 – 5) 2.5 (2 – 3.5) t1/2 (h) 11.08 (16.94) - Effect of food: LYSTEDA may be administered without regard to meals. A single dose administration (two 650 mg tablets) of LYSTEDA with food increased both Cmax and AUC by 7% and 16%, respectively.
Distribution
Tranexamic acid is 3% bound to plasma proteins with no apparent binding to albumin. Tranexamic acid is distributed with an initial volume of distribution of 0.18 L/kg and steady-state apparent volume of distribution of 0.39 L/kg.
Tranexamic acid crosses the placenta. The concentration in cord blood after an intravenous injection of 10 mg/kg to pregnant women is about 30 mg/L, as high as in the maternal blood.
Tranexamic acid concentration in cerebrospinal fluid is about one tenth of the plasma concentration.
The drug passes into the aqueous humor of the eye achieving a concentration of approximately one tenth of plasma concentrations.
Excretion
Tranexamic acid is eliminated by urinary excretion primarily via glomerular filtration with more than 95% of the dose excreted unchanged. Excretion of tranexamic acid is about 90% at 24 hours after intravenous administration of 10 mg/kg. Most elimination post intravenous administration occurred during the first 10 hours, giving an apparent elimination half-life of approximately 2 hours. The mean terminal half-life of LYSTEDA is approximately 11 hours. Plasma clearance of tranexamic acid is 110-116 mL/min.
Specific Populations
Pregnancy (Category B)
LYSTEDA is not indicated for use in pregnant women. Tranexamic acid is known to cross the placenta and appears in cord blood at concentrations approximately equal to maternal concentration. There are no adequate and well-controlled studies in pregnant women [see Use in Specific Populations (8.1)].
Nursing Mothers
Tranexamic acid is present in the mother's milk at a concentration of about one hundredth of the corresponding serum concentrations. LYSTEDA should be used during lactation only if clearly needed [see Use in Specific Populations (8.3)].
Pediatric Use
LYSTEDA is indicated for women of reproductive age and is not intended for use in premenarcheal girls.
In a randomized, single dose, two-way crossover study of two dose levels (650 mg and 1,300 mg [two 650 mg tablets]), pharmacokinetics of tranexamic acid was evaluated in 20 female adolescents (12 to 16 years of age) with heavy menstrual bleeding. The Cmax and AUC values after a single oral dose of 650 mg in the adolescent females were 32 – 36% less than those after a single oral dose of 1,300 mg in the adolescent females. The Cmax and AUC values after a single oral dose of 1300 mg in the adolescent females were 20 – 25% less than those in the adult females given the same dose in a separate study. [See Use in Specific Populations (8.4)]
Geriatric Use
LYSTEDA is indicated for women of reproductive age and is not intended for use by postmenopausal women.
Renal Impairment
The effect of renal impairment on the disposition of LYSTEDA has not been evaluated. Urinary excretion following a single intravenous injection of tranexamic acid declines as renal function decreases. Following a single 10 mg/kg intravenous injection of tranexamic acid in 28 patients, the 24-hour urinary fractions of tranexamic acid with serum creatinine concentrations 1.4 – 2.8, 2.8 – 5.7, and greater than 5.7 mg/dL were 51, 39, and 19%, respectively. The 24-hour tranexamic acid plasma concentrations for these patients demonstrated a direct relationship to the degree of renal impairment. Therefore, dose adjustment is needed in patients with renal impairment [see Dosage and Administration (2.2)].
Hepatic Impairment
The effect of hepatic impairment on the disposition of LYSTEDA has not been evaluated. One percent and 0.5 percent of an oral dose are excreted as a dicarboxylic acid and acetylated metabolite, respectively. Because only a small fraction of the drug is metabolized, no dose adjustment is needed in patients with hepatic impairment.
Hormonal Contraceptives
Because LYSTEDA is antifibrinolytic, concomitant use of hormonal contraception and LYSTEDA may further exacerbate the increased thrombotic risk associated with combination hormonal contraceptives. For this reason, concomitant use of LYSTEDA with combination hormonal contraceptives is contraindicated [see Contraindications (4), Warnings and Precautions (5.1) and Drug Interactions (7.1)].
Factor IX Complex Concentrates or Anti-inhibitor Coagulant Concentrates
LYSTEDA is not recommended in patients taking either Factor IX complex concentrates or anti-inhibitor coagulant concentrates because the risk of thrombosis may be increased [see Warnings and Precautions (5.1) and Drug Interactions (7.3)].
Tissue Plasminogen Activators
Concomitant therapy with tissue plasminogen activators may decrease the efficacy of both LYSTEDA and tissue plasminogen activators. Therefore, exercise caution if a patient taking LYSTEDA therapy requires tissue plasminogen activators [see Drug Interactions (7.2)].
All-Trans Retinoic Acid (Oral Tretinoin)
In a study involving 28 patients with acute promyelocytic leukemia who were given either orally administered all-trans retinoic acid plus intravenously administered tranexamic acid, all-trans retinoic acid plus chemotherapy, or all-trans retinoic acid plus tranexamic acid plus chemotherapy, all 4 patients who were given all-trans retinoic acid plus tranexamic acid died, with 3 of the 4 deaths due to thrombotic complications. It appears that the procoagulant effect of all-trans retinoic acid may be exacerbated by concomitant use of tranexamic acid. Therefore, exercise caution when prescribing LYSTEDA to patients with acute promyelocytic leukemia taking all-trans retinoic acid [see Warnings and Precautions (5.1) and Drug Interactions (7.4)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity studies with tranexamic acid in male mice at doses as high as 6 times the recommended human dose of 3900 mg/day showed an increased incidence of leukemia which may have been related to treatment. Female mice were not included in this experiment.
The dose multiple referenced above is based on body surface area (mg/m2). Actual daily dose in mice was up to 5000 mg/kg/day in food.
Hyperplasia of the biliary tract and cholangioma and adenocarcinoma of the intrahepatic biliary system have been reported in one strain of rats after dietary administration of doses exceeding the maximum tolerated dose for 22 months. Hyperplastic, but not neoplastic, lesions were reported at lower doses. Subsequent long-term dietary administration studies in a different strain of rat, each with an exposure level equal to the maximum level employed in the earlier experiment, have failed to show such hyperplastic/neoplastic changes in the liver.
Mutagenesis
Tranexamic acid was neither mutagenic nor clastogenic in the in vitro Bacterial Reverse Mutation Assay (Ames test), in vitro chromosome aberration test in Chinese hamster cells, and in in vivo chromosome aberration tests in mice and rats.
Impairment of Fertility
Reproductive studies performed in mice, rats and rabbits have not revealed any evidence of impaired fertility or adverse effects on the fetus due to tranexamic acid.
In a rat embryo-fetal developmental toxicity study, tranexamic acid had no adverse effects on embryo-fetal development when administered during the period of organogenesis (from gestation days 6 through 17) at doses 1, 2 and 4 times the recommended human oral dose of 3900 mg/day. In a perinatal-postnatal study in rats, tranexamic acid had no adverse effects on pup viability, growth or development when administered from gestation day 6 through postnatal day 20 at doses 1, 2 and 4 times the recommended human oral dose of 3900 mg/day.
The dose multiples referenced above are based on body surface area (mg/m2). Actual daily doses in rats were 300, 750 or 1500 mg/kg/day.
13.2 Animal Toxicology and/or Pharmacology
Ocular Effects
In a 9-month toxicology study, dogs were administered tranexamic acid in food at doses of 0, 200, 600, or 1200 mg/kg/day. These doses are approximately 2, 5, and 6 times, respectively, the recommended human oral dose of 3900 mg/day based on AUC. At 6 times the human dose, some dogs developed reversible reddening and gelatinous discharge from the eyes. Ophthalmologic examination revealed reversible changes in the nictitating membrane/conjunctiva. In some female dogs, the presence of inflammatory exudate over the bulbar conjunctival mucosa was observed. Histopathological examinations did not reveal any retinal alteration. No adverse effects were observed at 5 times the human dose.
In other studies, focal areas of retinal degeneration were observed in cats, dogs and rats following oral or intravenous tranexamic acid doses at 6-40 times the recommended usual human dose based on mg/m2 (actual animal doses between 250-1600 mg/kg/day).
-
14 CLINICAL STUDIES
The efficacy and safety of LYSTEDA in the treatment of heavy menstrual bleeding (HMB) was demonstrated in one 3-cycle treatment and one 6-cycle treatment, randomized, double-blind, placebo-controlled study [see Adverse Reactions (6.1)]. In these studies, HMB was defined as an average menstrual blood loss of ≥ 80 mL as assessed by alkaline hematin analysis of collected sanitary products over two baseline menstrual cycles. Subjects were 18 to 49 years of age with a mean age of approximately 40 years, had cyclic menses every 21-35 days, and a BMI of approximately 32 kg/m2. On average, subjects had an HMB history of approximately 10 years and 40% had fibroids as determined by transvaginal ultrasound. Approximately 70% were Caucasian, 25% were Black, and 5% were Asian, Native American, Pacific Islander, or Other. Seven percent (7%) of all subjects were of Hispanic origin.
In these studies, the primary outcome measure was menstrual blood loss (MBL), measured using the alkaline hematin method. The endpoint was change from baseline in MBL, calculated by subtracting the mean MBL during treatment from the mean pretreatment MBL.
The key secondary outcome measures were based on specific questions concerning limitations in social or leisure activities (LSLA) and limitations in physical activities (LPA). Large stains (soiling beyond the undergarment) were also included as a key secondary outcome measure.
14.1 Three-Cycle Treatment Study
This study compared the effects of two doses of LYSTEDA (1950 mg and 3900 mg given daily for up to 5 days during each menstrual period) versus placebo on MBL over a 3-cycle treatment duration. Of the 294 evaluable subjects, 115 LYSTEDA 1950 mg/day subjects, 112 LYSTEDA 3900 mg/day subjects and 67 placebo subjects took at least one dose of study drug and had post-treatment data available.
Results are shown in Table 4. MBL was statistically significantly reduced in patients treated with 3900 mg/day LYSTEDA compared to placebo. Study success also required achieving a reduction in MBL that was determined to be clinically meaningful to the subjects. The 1950 mg/day LYSTEDA dose did not meet the criteria for success.
Table 4. Mean Reduction from Baseline in MBL Treatment Arm N Baseline Mean MBL (mL) Least Squares Mean Reduction in MBL (mL) Percent Reduction in MBL - * p<0.001 versus placebo
LYSTEDA 3900 mg/day 112 169 65* 39% LYSTEDA 1950 mg/day 115 178 44 25% Placebo 67 154 7 5% LYSTEDA also statistically significantly reduced limitations on social, leisure, and physical activities in the 3900 mg/day dose group compared to placebo (see Table 5). No statistically significant treatment difference was observed in response rates on the number of large stains.
Table 5: Secondary Outcomes in 3-Cycle Study Outcome Measure N Baseline Mean * Least Squares Mean Reduction † - * Response categories: 1=not at all limited; 2=slightly limited; 3=moderately limited; 4=quite a bit limited; 5=extremely limited
- † Positive means reflect an improvement from baseline.
- ‡ p-value <0.05 versus placebo
- § Responders are defined as subjects who experienced a reduction from baseline in frequency of large stains.
- ¶ Non-significant difference versus placebo
Social and Leisure Activities 3900 mg/day LYSTEDA 112 3.00 0.98‡ Placebo 66 2.85 0.39 Physical Activities 3900 mg/day LYSTEDA 112 3.07 0.94‡ Placebo 66 2.96 0.34 N Responders § Reduction in Large Stains 3900 mg/day LYSTEDA 111 64%¶ Placebo 67 52% 14.2 Six-Cycle Treatment Study
This study compared the effects of LYSTEDA 3900 mg/day given daily for up to 5 days during each menstrual period versus placebo on MBL over a 6-cycle treatment duration. Of the 187 evaluable subjects, 115 LYSTEDA subjects and 72 placebo subjects took at least one dose of study drug and had post-treatment data available.
Results are shown in Table 6. MBL was statistically significantly reduced in patients treated with 3900 mg/day LYSTEDA compared to placebo. Study success also required achieving a reduction in MBL that was determined to be clinically meaningful to the subjects.
Table 6. Mean Reduction from Baseline in MBL Treatment Arm N Baseline Mean MBL (mL) Least Squares Mean Reduction in MBL (mL) Percent Reduction in MBL - * p<0.001 versus placebo
LYSTEDA 3900 mg/day 115 172 66* 38% Placebo 72 153 18 12% Limitations on social, leisure, and physical activities were also statistically significantly reduced in the LYSTEDA group compared to placebo (see Table 7). No statistically significant treatment difference was observed in response rates on the number of large stains.
Table 7. Secondary Outcomes in 6-Cycle Study Outcome Measure N Baseline Mean * Least Squares Mean Reduction † - * Response categories: 1=not at all limited; 2=slightly limited; 3=moderately limited; 4=quite a bit limited; 5=extremely limited
- † Positive means reflect an improvement from baseline
- ‡ p-value <0.05 versus placebo
- § Responders are defined as subjects who experienced a reduction from baseline in frequency of large stains
- ¶ Non-significant difference versus placebo
Social and Leisure Activities 3900 mg/day LYSTEDA 115 2.92 0.85‡ Placebo 72 2.74 0.44 Physical Activities 3900 mg/day LYSTEDA 115 3.05 0.87‡ Placebo 72 2.90 0.40 N Responders § Reduction in Large Stains 3900 mg/day LYSTEDA 115 57%¶ Placebo 72 51% 14.3 MBL Results over Time
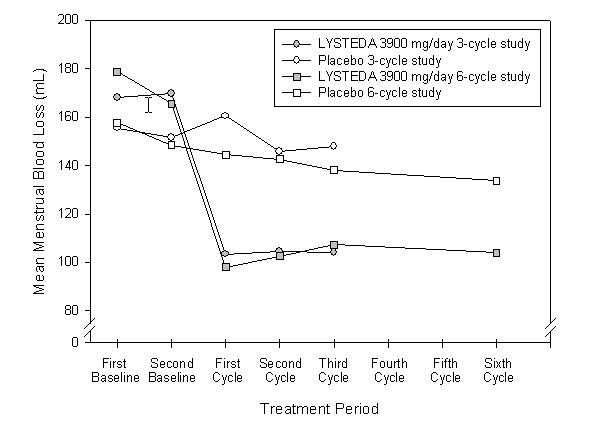
The efficacy of LYSTEDA 3900 mg/day over 3 menstrual cycles and over 6 menstrual cycles was demonstrated versus placebo in the double-blind, placebo-controlled efficacy studies (see Figure 1). The change in MBL from baseline was similar across all post-baseline treatment cycles.
Figure 1: MBL Levels over Duration of Therapy
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information)
Instruct patients that the usual schedule is to take two tablets with liquids, three times a day during menstruation. Patients should be instructed not to exceed 3 doses (6 tablets) in a 24-hour period or to take for more than 5 days in any menstrual cycle.
Inform patients that they should immediately stop LYSTEDA if they notice any eye symptoms or change in their vision. Instruct them to report any such problems promptly to their physician and to follow-up with an ophthalmologist for a complete ophthalmic evaluation, including dilated retinal examination of the retina.
Inform patients that they should stop LYSTEDA and seek immediate medical attention if they notice symptoms of a severe allergic reaction (e.g., shortness of breath or throat tightening).
Instruct patients that common side effects of LYSTEDA include headache, sinus and nasal symptoms, back pain, abdominal pain, musculoskeletal pain, joint pain, muscle cramps, migraine, anemia and fatigue.
Advise patients to contact their healthcare provider if their heavy menstrual bleeding symptoms persist or worsen.
Remind patients to read the Patient Labeling carefully.
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
LYSTEDA (pronounced lye-sted-a) tranexamic acid, USP tabletsRead the Patient Information that comes with LYSTEDA before you start using the drug and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What is LYSTEDA?
LYSTEDA is a prescription medicine used to treat your heavy monthly period (menstruation) when your bleeding gets in the way of social, leisure and physical activities. LYSTEDA does not contain any hormones. On average, LYSTEDA has been shown to lower the amount of blood lost during your monthly period by about one-third, but it is not meant to stop your period.
LYSTEDA is taken only during your period and is not meant to treat pre-menstrual symptoms (symptoms that occur before your bleeding starts). LYSTEDA does not affect your fertility and cannot be used as birth control. LYSTEDA does not protect you against diseases that you may get if you have unprotected sex.
LYSTEDA has not been studied in adolescents younger than 18 years of age.
LYSTEDA is not for women who have already gone through menopause (post-menopausal).
Who should not take LYSTEDA?
Do not take LYSTEDA if you:
- Are using a form of birth control that contains estrogen and a progestin (like a birth control pill, patch, or vaginal ring). Ask your healthcare provider before taking LYSTEDA if you are not sure if your birth control method contains estrogen and a progestin.
- Currently have a blood clot
- Have ever had a blood clot
- Have been told that you are at risk of having a blood clot
- Are allergic to LYSTEDA or tranexamic acid
What should I tell my healthcare provider before taking LYSTEDA?
Before taking LYSTEDA, tell your healthcare provider about all of your medical conditions, including whether:
- You have ever had a blood clot or been told that you are at risk of having a blood clot
- You are using a form of birth control that contains estrogen and a progestin (like a birth control pill, patch, or vaginal ring). Using hormonal birth control along with LYSTEDA may increase your chance of having a serious blood clot, stroke, or heart attack. For this reason, do not use LYSTEDA if you use a form of birth control that contains estrogen and a progestin.
- You are pregnant or think you may be pregnant
- You are breastfeeding or plan to breast-feed. LYSTEDA can pass into your milk. Talk to your healthcare provider about the best way to feed your baby if you take LYSTEDA.
- The time between the start of your periods is less than 21 days or more than 35 days
- You have any other medical conditions
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. LYSTEDA and other medicines can affect each other, causing side effects. LYSTEDA can affect the way other medicines work and other medicines can affect how LYSTEDA works.
Especially tell your healthcare provider if you take:
- Birth control pills or other hormonal birth control
- Medicines used to help your blood form clots
- Medicines used to break up blood clots
- Any medicines to treat leukemia
Ask your healthcare provider if you are not sure if your medicine is one that is described above.
How should I take LYSTEDA?
- Take LYSTEDA exactly as your healthcare provider tells you.
- Do not take LYSTEDA until your period has started.
- Do not take LYSTEDA for more than 5 days in a row.
- Do not take LYSTEDA when you do not have your period.
- Once your period has started, take 2 tablets of LYSTEDA three times per day (e.g., in the morning, afternoon, and evening).
- LYSTEDA tablets should be swallowed whole and not chewed or broken apart.
- LYSTEDA may be taken with or without food.
- Do not take more than 6 tablets of LYSTEDA in a day. If you take more than 6 tablets, call your healthcare provider.
- If you miss a dose, take it when you remember, and then take your next dose at least six hours later. Do not take more than two tablets at a time to make up for missed doses.
- If LYSTEDA does not help to lessen bleeding with your periods after 2 cycles or seems to stop working, talk to your healthcare provider.
What are the possible side effects of LYSTEDA?
LYSTEDA can cause serious side effects, including:
- Blood clots. You may have a higher risk of having serious blood clots if you take LYSTEDA with:
- medicines used to help your blood form clots
- some medicines used to treat leukemia
- Eye changes. Stop taking LYSTEDA and promptly report any eye problems you have while taking LYSTEDA. Your doctor will refer you to an eye doctor who will examine your eyes.
- Allergic reaction. If you have severe shortness of breath and your throat feels tight, stop taking LYSTEDA and get medical care right away.
The most common side effects of LYSTEDA include:
- Headaches
- Sinus and nasal problems
- Back pain
- Pain in your abdomen
- Pain in your muscles or joints
- Anemia
- Fatigue
Tell your healthcare provider if you have any side effect that bothers you or does not go away.
These are not all of the possible side effects of LYSTEDA. For more information, ask your healthcare provider or pharmacist.
If you notice a change in your usual bleeding pattern that worries you, or your heavy bleeding continues, contact your healthcare provider right away. This may be a sign of a more serious condition.
Call your healthcare provider for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088. You may also report side effects to Ferring Pharmaceuticals Inc. at 1-888-FERRING (1-888-337-7464).
How should I store LYSTEDA?
Store LYSTEDA at room temperature between 59°F to 86°F (15°C to 30°C).
Keep LYSTEDA and all medicines out of the reach of children.
General information about LYSTEDA
Medicines are sometimes prescribed for conditions that are not mentioned in Patient Information Leaflets. Do not use LYSTEDA for a condition for which it was not prescribed. Do not give LYSTEDA to other people, even if they have the same symptoms that you have. It may harm them.
This patient information leaflet summarizes the most important information about LYSTEDA. If you would like more information about LYSTEDA, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about LYSTEDA that is written for healthcare professionals. For more information, go to www.lysteda.com or call 1-888-FERRING (1-888-337-7464).
What are the ingredients of LYSTEDA?
Active ingredient: tranexamic acid
Inactive ingredients: microcrystalline cellulose, colloidal silicon dioxide, pregelatinized corn starch, povidone, hypromellose, stearic acid, and magnesium stearate.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Manufactured for:
Ferring Pharmaceuticals Inc.
Parsippany, NJ 07054By: Mikart, LLC
Atlanta, GA 30318Rev. 04/2019
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 650 mg Tablet Bottle Label
Rx only
NDC: 55566-2110-2Lysteda®
(tranexamic acid, USP) tablets650 mg
30 tablets
-
INGREDIENTS AND APPEARANCE
LYSTEDA
tranexamic acid tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 55566-2110 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRANEXAMIC ACID (UNII: 6T84R30KC1) (TRANEXAMIC ACID - UNII:6T84R30KC1) TRANEXAMIC ACID 650 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) STEARIC ACID (UNII: 4ELV7Z65AP) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white Score score with uneven pieces Shape OVAL Size 17mm Flavor Imprint Code FP650 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55566-2110-2 30 in 1 BOTTLE; Type 0: Not a Combination Product 05/17/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022430 05/17/2010 Labeler - Ferring Pharmaceuticals Inc. (103722955) Establishment Name Address ID/FEI Business Operations Mikart, LLC 030034847 manufacture(55566-2110) , pack(55566-2110) Establishment Name Address ID/FEI Business Operations Kyowa Pharma Chemical Co. Ltd. 690852371 API MANUFACTURE(55566-2110) , ANALYSIS(55566-2110)
Trademark Results [Lysteda]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LYSTEDA 77641089 3857576 Live/Registered |
FERRING B.V. 2008-12-30 |
 LYSTEDA 77560854 3931482 Live/Registered |
Ferring B.V. 2008-09-02 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.