NALOXONE HYDROCHLORIDE spray, metered
Naloxone Hydrochloride by
Drug Labeling and Warnings
Naloxone Hydrochloride by is a Otc medication manufactured, distributed, or labeled by Actavis Pharma, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (in each spray)
- Purpose
- Uses
-
Directions
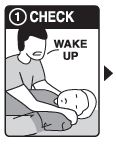
Step 1: CHECK if you suspect an overdose:
- CHECK for a suspected overdose: the person will not wake up or is very sleepy or not breathing well
- yell “Wake up!”
- shake the person gently
- if the person is not awake, go to Step 2
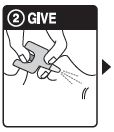
Step 2: GIVE 1stdose in the nose
- HOLD the nasal spray device with your thumb on the bottom of the plunger
- INSERT the nozzle into either NOSTRIL
- PRESS the plunger firmly to give the 1st dose
- 1 nasal spray device contains 1 dose

Step 3: CALL
- CALL 911immediately after giving the 1st dose
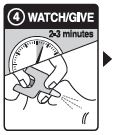
Step 4: WATCH & GIVE
- WAIT2-3 minutes after the 1stdose to give the medicine time to work
- if the person wakes up: Go to Step 5
- if the person does not wake up:
- CONTINUE TO GIVE doses every 2-3 minutes until the person wakes up
- it is safe to keep giving doses

Step 5: STAY
- STAY until ambulance arrives: even if the person wakes up
- GIVE another dose if the person becomes very sleepy again
- You may need to give all the doses in the pack
- Warning
- Other information
- Inactive ingredients
- Questions?
- KEEP OUT OF REACH OF CHILDREN
- KEEP OUT OF REACH OF CHILDREN
-
PACKAGE LABEL PRINCIPAL DISPLAY PANEL
NOT FOR RESALE
FOR NON-RETAIL
DISTRIBUTIONCompare to the active
ingredient in NARCAN®*NDC: 0591-3871-99
Naloxone Hydrochloride
Nasal Spray
4mgEmergency Treatment of
Opioid OverdoseDesigned to Rapidly Reverse the
Effects of a Life-Threatening
Opioid EmergencyFor use in nose only
2 Single-Dose Nasal Spray Devices
0.003 fl oz (0.1 mL) each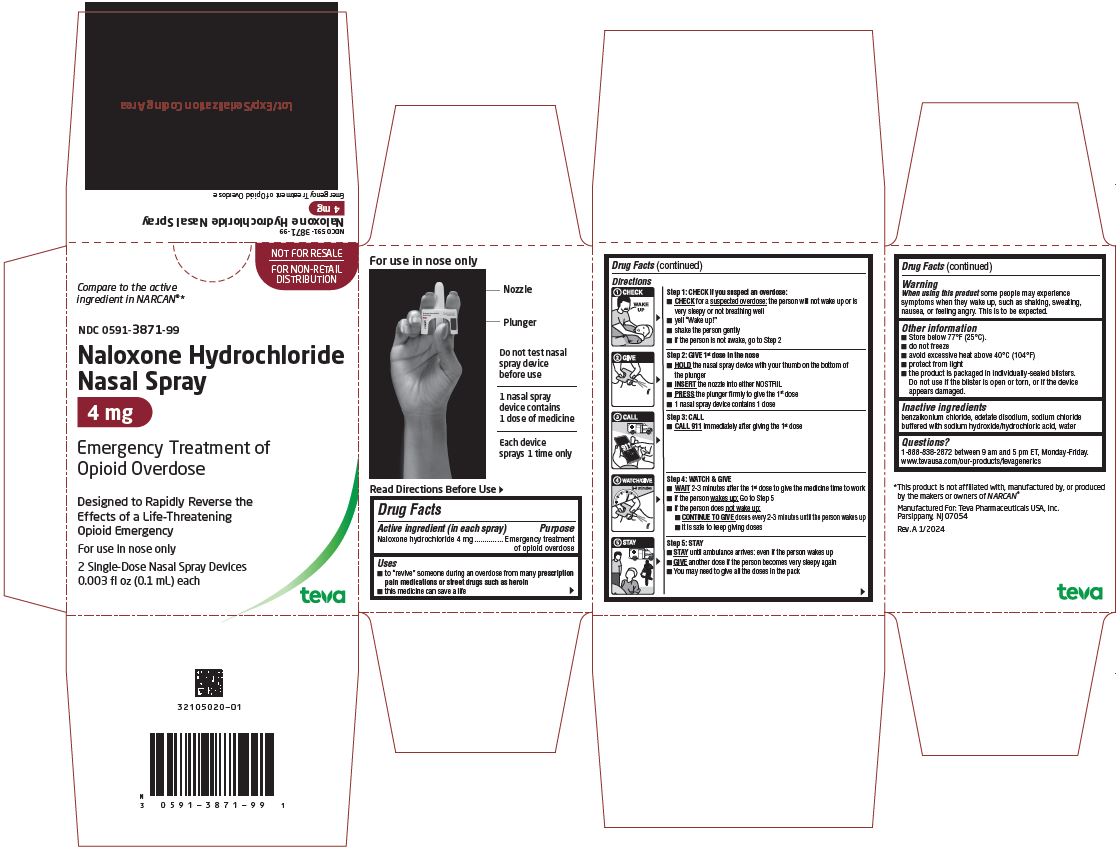
-
INGREDIENTS AND APPEARANCE
NALOXONE HYDROCHLORIDE
naloxone hydrochloride spray, meteredProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0591-3871 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NALOXONE HYDROCHLORIDE (UNII: F850569PQR) (NALOXONE - UNII:36B82AMQ7N) NALOXONE HYDROCHLORIDE 4 mg in 0.1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0591-3871-99 2 in 1 CARTON 01/17/2025 1 NDC: 0591-3871-54 1 in 1 BLISTER PACK 1 0.1 mL in 1 VIAL, SINGLE-DOSE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209522 01/17/2025 Labeler - Actavis Pharma, Inc. (119723554)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.