CVS HAND SANITIZER- alcohol gel
CVS Hand Sanitizer by
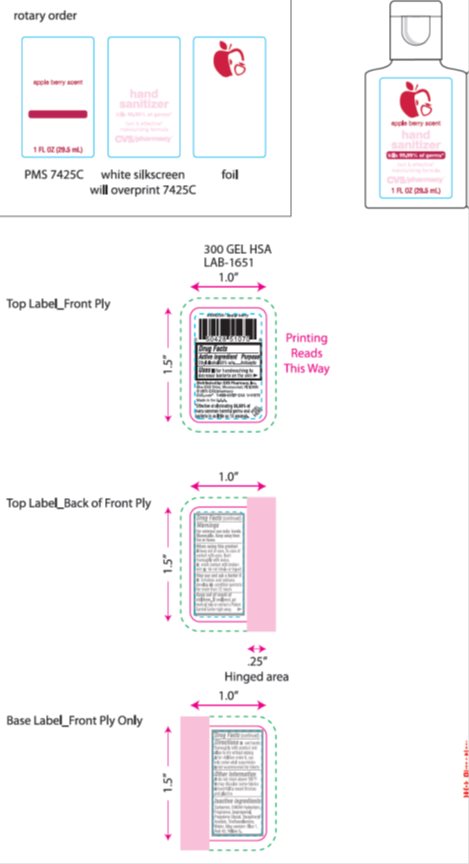
Drug Labeling and Warnings
CVS Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by CVS, OraLabs. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient
- Purpose
- Keep Out of Reach of Children
- Uses
-
Warnings
For external use only: Hands. Flammable. Keep away from fire or flame. When using this product: keep out of eyes. In case of contact with eyes, flush thoroughly with water. Avoid contact with broken skin. Do not inhale or ingest. Stop use and ask a doctor: if irritation and redness develop. Condition persists for more than 72 hours.
- Directions
- Inactive Ingredients
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
CVS HAND SANITIZER
alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69842-554 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 58.10 mg in 1 g Inactive Ingredients Ingredient Name Strength .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 0.010 mg in 1 g CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) .310 mg in 1 g PROPYLENE GLYCOL (UNII: 6DC9Q167V3) .500 mg in 1 g Product Characteristics Color PINK Score Shape Size Flavor APPLE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69842-554-01 1 g in 1 CONTAINER; Type 0: Not a Combination Product 08/17/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 08/17/2015 Labeler - CVS (062312574) Registrant - OraLabs (801824756) Establishment Name Address ID/FEI Business Operations OraLabs 801824756 MANUFACTURE(69842-554) , LABEL(69842-554)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.