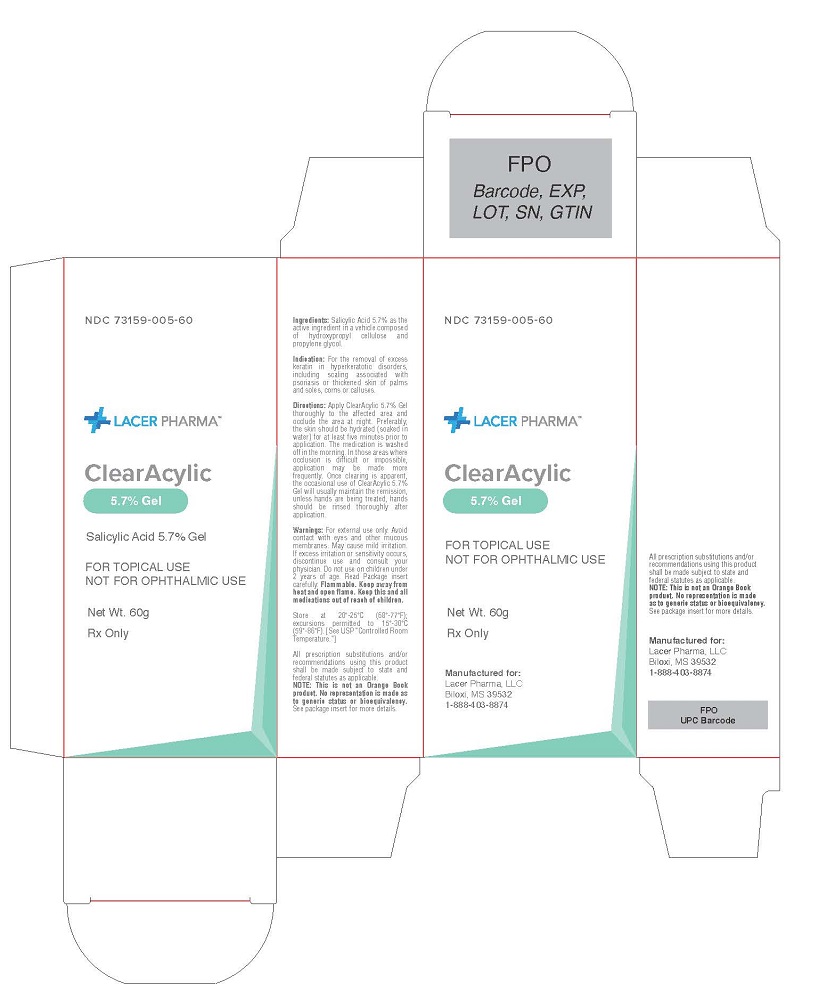
ClearAcylic 5.7% Gel For Topical Use Not for Opthalmic Use
ClearAcylic by
Drug Labeling and Warnings
ClearAcylic by is a Prescription medication manufactured, distributed, or labeled by Lacer Pharma, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CLEARACYLIC- salycilic acid gel
Lacer Pharma, LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
ClearAcylic
5.7% Gel
For Topical Use
Not for Opthalmic Use
Indication
For the removal of excess keratin in hyperkeratotic disorders, including scaling associated with psoriasis or thickened skin of palms and soles, corns or calluses.
Directions
Apply ClearAcylic 5.7% Gel thoroughly to the affected area and occlude the area at night. Preferably, the skin should be hydrated (soaked in water) for at least five minutes prior to application. The medication is washed off in the morning. In those areas where occlusion is difficult or impossible, application may be made more frequently. Once clearing is apparent, the occasional use of ClearAcylic 5.7% Gel will usually maintain the remission, unless hands are being treated, hands should be rinsed thoroughly after application.
Warnings
For external use only. Avoid contact with eyes and other mucous membranes. May cause mild irritation. If excess irritation or sensitivity occurs, discontinue use and consult your physician. Do not use on children under 2 years of age. Read Package insert carefully. Flammable. Keep away from heat and open flame. Keep this and all medications out of reach of children.
ClearAcylic
5.7% Gel
FOR TOPICAL USE
NOT FOR OPHTHALMIC USE
NDC: 73159-005-60
Net Weight: 60 g
Tube label

Carton Label
| CLEARACYLIC
salycilic acid gel |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Lacer Pharma, LLC (117072266) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.