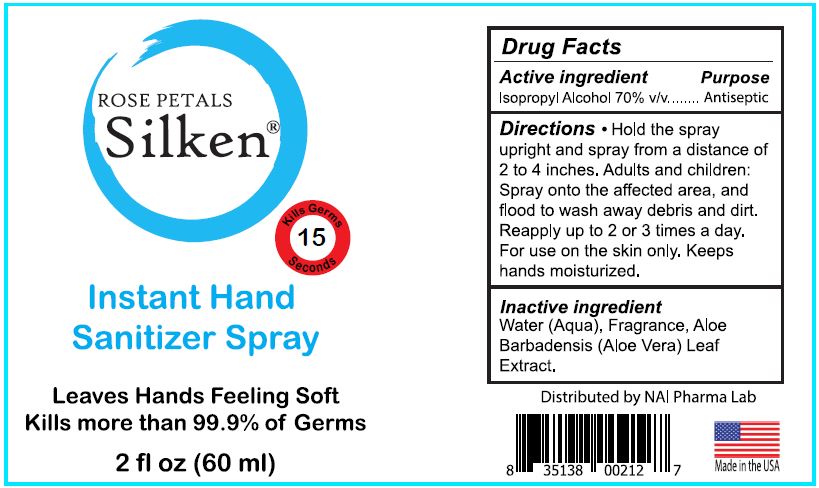
COSMOBEAUTI - ROSE PETALS SILKEN INSTANT HAND SANITIZER SPRAY (69077-104) - DELIST
SILKEN INSTANT HAND SANITIZER by
Drug Labeling and Warnings
SILKEN INSTANT HAND SANITIZER by is a Otc medication manufactured, distributed, or labeled by COSMOBEAUTI LABORATORIES & MANUFACTURING, INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SILKEN INSTANT HAND SANITIZER- isopropyl alcohol spray
COSMOBEAUTI LABORATORIES & MANUFACTURING, INC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
COSMOBEAUTI - ROSE PETALS SILKEN INSTANT HAND SANITIZER SPRAY (69077-104) - DELIST
DIRECTIONS
HOLD THE SPRAY UPRIGHT AND SPRAY FROM A DISTANCE OF 2 TO 4 INCHES. ADULTS AND CHILDREN: SPRAY ONTO THE AFFECTED AREA, AND FLOOD TO WASH AWAY DEBRIS AND DIRT. REAPPLY UP TO 2 TO 3 TIMES A DAY.
| SILKEN INSTANT HAND SANITIZER
isopropyl alcohol spray |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - COSMOBEAUTI LABORATORIES & MANUFACTURING, INC. (021687376) |
Revised: 3/2023
Document Id: f67cc70f-fc1c-4973-e053-2a95a90adce7
Set id: 49de9157-904a-497d-a879-7613d101d1ae
Version: 3
Effective Time: 20230309
COSMOBEAUT
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.