REZLIDHIA- olutasidenib capsule
REZLIDHIA by
Drug Labeling and Warnings
REZLIDHIA by is a Prescription medication manufactured, distributed, or labeled by Rigel Pharmaceuticals, Inc., Catalent Greenville, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use REZLIDHIA safely and effectively. See full prescribing information for REZLIDHIA.
REZLIDHIA® (olutasidenib) capsules, for oral use
Initial U.S. Approval: 2022WARNING: DIFFERENTIATION SYNDROME
See full prescribing information for complete boxed warning.
- Differentiation syndrome, which can be fatal, can occur with REZLIDHIA treatment.
- If differentiation syndrome is suspected, withhold REZLIDHIA and initiate corticosteroids and hemodynamic monitoring until symptom resolution. (5.1)
INDICATIONS AND USAGE
REZLIDHIA is an isocitrate dehydrogenase-1 (IDH1) inhibitor indicated for the treatment of adult patients with relapsed or refractory acute myeloid leukemia (AML) with a susceptible IDH1 mutation as detected by an FDA-approved test. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Capsules: 150 mg (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
The most common (≥20%) adverse reactions, including laboratory abnormalities, are aspartate aminotransferase increased, alanine aminotransferase increased, potassium decreased, sodium decreased, alkaline phosphatase increased, nausea, creatinine increased, fatigue/malaise, arthralgia, constipation, lymphocytes increased, bilirubin increased, leukocytosis, uric acid increased, dyspnea, pyrexia, rash, lipase increased, mucositis, diarrhea and transaminitis. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Rigel Pharmaceuticals, Inc. at 1-800-983-1329 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
Lactation: Advise not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 4/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: DIFFERENTIATION SYNDROME
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
2.2 Recommended Dosage
2.3 Monitoring and Dosage Modifications for Adverse Reactions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Differentiation Syndrome
5.2 Hepatotoxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on Olutasidenib
7.2 Effect of Olutasidenib on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Acute Myeloid Leukemia
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: DIFFERENTIATION SYNDROME
Differentiation syndrome, which can be fatal, can occur with REZLIDHIA treatment. Symptoms may include dyspnea, pulmonary infiltrates/pleuropericardial effusion, kidney injury, hypotension, fever, and weight gain.
If differentiation syndrome is suspected, withhold REZLIDHIA and initiate treatment with corticosteroids and hemodynamic monitoring until symptom resolution [see Warnings and Precautions (5.1)].
-
1 INDICATIONS AND USAGE
Relapsed or Refractory Acute Myeloid Leukemia
REZLIDHIA is indicated for the treatment of adult patients with relapsed or refractory acute myeloid leukemia (AML) with a susceptible isocitrate dehydrogenase-1 (IDH1) mutation as detected by an FDA-approved test [see Dosage and Administration (2.1), Clinical Pharmacology (12.1), and Clinical Studies (14.1)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
Select patients for the treatment of relapsed or refractory AML with REZLIDHIA based on the presence of IDH1 mutations in blood or bone marrow [see Clinical Trials (14.1)]. Information on FDA- approved tests for the detection of IDH1 mutations in AML is available at http://www.fda.gov/CompanionDiagnostics.
2.2 Recommended Dosage
The recommended dosage of REZLIDHIA is 150 mg taken orally twice daily until disease progression or unacceptable toxicity. Administer REZLIDHIA capsules orally about the same time each day. Do not administer 2 doses within 8 hours. Take on an empty stomach at least 1 hour before or 2 hours after a meal [see Clinical Pharmacology (12.3)]. For patients without disease progression or unacceptable toxicity, treat for a minimum of 6 months to allow time for clinical response.
Swallow REZLIDHIA capsules whole. Do not break, open, or chew the capsules. If a dose of REZLIDHIA is vomited, do not administer a replacement dose; wait until the next scheduled dose is due. If a dose of REZLIDHIA is missed or not taken at the usual time, administer the dose as soon as possible and at least 8 hours prior to the next scheduled dose. Return to the normal schedule the following day.
2.3 Monitoring and Dosage Modifications for Adverse Reactions
Assess blood counts, and blood chemistries including liver function tests prior to initiation of REZLIDHIA, at least once weekly for the first two months; once every other week for the third month; once in the fourth month, and once every other month for the duration of therapy. Manage any abnormalities promptly [see Warnings and Precautions (5.1 and 5.2) and Adverse Reactions (6.1)].
Interrupt dosing or reduce dose for toxicities. See Table 1 for dosage modification guidelines.
Table 1: Recommended Dosage Modifications for REZLIDHIA - * Grade 1 is mild, Grade 2 is moderate, Grade 3 is severe, Grade 4 is life-threatening. Severity as defined by National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE version 4.03).
Adverse Reactions Recommended Action Differentiation Syndrome
[see Warnings and Precautions (5.1)]- If differentiation syndrome is suspected, withhold REZLIDHIA until signs and symptoms improve.
- Administer systemic corticosteroids and initiate hemodynamic monitoring until symptom resolution and for a minimum of 3 days.
- Resume REZLIDHIA at 150 mg twice daily after resolution of differentiation syndrome.
- If a recurrence of differentiation syndrome is suspected, withhold REZLIDHIA and institute treatment per above guidance. After resolution of symptoms, REZLIDHIA may be resumed at a reduced dose of 150 mg once daily for a minimum of 7 days, after which it can be increased to 150 mg twice daily.
Noninfectious leukocytosis
[see Adverse Reactions (6.1)]- Initiate treatment with hydroxyurea, as per standard practices. Taper hydroxyurea only after leukocytosis improves or resolves.
Grade 3* hepatotoxicity
[see Warnings and Precautions (5.2)]- Withhold REZLIDHIA and monitor liver function tests, twice per week, until laboratory values have returned to baseline or Grade 1* toxicity.
- Resume REZLIDHIA at a reduced dose of 150 mg once daily and continue monitoring; may increase to 150 mg twice daily if hepatotoxicity resolves to baseline for at least 28 days.
- If hepatotoxicity (Grade 3) recurs at 150 mg once daily, discontinue REZLIDHIA.
Grade 4* hepatotoxicity or AST or ALT >3x ULN and total bilirubin >2x ULN and alkaline phosphatase <2x ULN in the absence of a clear alternative explanation
[see Warnings and Precautions (5.2)]- Permanently discontinue REZLIDHIA
Other Grade 3* or higher toxicity considered related to treatment
[see Adverse Reactions (6.1)] - 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Differentiation Syndrome
REZLIDHIA can cause differentiation syndrome. In the clinical trial of REZLIDHIA in patients with relapsed or refractory AML, differentiation syndrome occurred in 16% (25/153) of patients, with grade 3 or 4 differentiation syndrome occurring in 8% of patients treated, and fatalities in 1% of patients [see Adverse Reactions (6.1)]. Differentiation syndrome is associated with rapid proliferation and differentiation of myeloid cells and may be life-threatening or fatal. Symptoms of differentiation syndrome in patients treated with REZLIDHIA included leukocytosis, dyspnea, pulmonary infiltrates/pleuropericardial effusion, kidney injury, fever, edema, pyrexia, and weight gain. Of the 25 patients who experienced differentiation syndrome, 19 (76%) recovered after treatment or after dosage interruption of REZLIDHIA. Differentiation syndrome occurred as early as 1 day and up to 18 months after REZLIDHIA initiation and has been observed with or without concomitant leukocytosis.
If differentiation syndrome is suspected, temporarily withhold REZLIDHIA and initiate systemic corticosteroids (e.g., dexamethasone 10 mg IV every 12 hours) for a minimum of 3 days and until resolution of signs and symptoms. If concomitant leukocytosis is observed, initiate treatment with hydroxyurea, as clinically indicated. Taper corticosteroids and hydroxyurea after resolution of symptoms. Differentiation syndrome may recur with premature discontinuation of corticosteroids and/or hydroxyurea treatment. Institute supportive measures and hemodynamic monitoring until improvement; withhold dose of REZLIDHIA and consider dose reduction based on recurrence [see Dosage and Administration (2.3)].
5.2 Hepatotoxicity
REZLIDHIA can cause hepatotoxicity, presenting as increased alanine aminotransferase (ALT), increased aspartate aminotransferase (AST), increased blood alkaline phosphatase, and/or elevated bilirubin. Of 153 patients with relapsed or refractory AML who received REZLIDHIA, hepatotoxicity occurred in 23% of patients; 13% experienced grade 3 or 4 hepatotoxicity [see Adverse Reactions (6.1)]. One patient treated with REZLIDHIA in combination with azacitidine in the clinical trial, a combination for which REZLIDHIA is not indicated, died from complications of drug-induced liver injury. The median time to onset of hepatotoxicity in patients with relapsed or refractory AML treated with REZLIDHIA was 1.2 months (range: 1 day to 17.5 months) after REZLIDHIA initiation, and the median time to resolution was 12 days (range: 1 day to 17 months). The most common hepatotoxicities were elevations of ALT, AST, blood alkaline phosphatase, and blood bilirubin.
Monitor patients frequently for clinical symptoms of hepatic dysfunction such as fatigue, anorexia, right upper abdominal discomfort, dark urine, or jaundice. Obtain baseline liver function tests prior to initiation of REZLIDHIA, at least once weekly for the first two months, once every other week for the third month, once in the fourth month, and once every other month for the duration of therapy. If hepatic dysfunction occurs, withhold, reduce, or permanently discontinue REZLIDHIA based on recurrence/severity [see Dosage and Administration (2.3)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Differentiation Syndrome [see Warnings and Precautions (5.1)]
- Hepatotoxicity [see Warnings and Precautions (5.2)]
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.1 Clinical Trials Experience
Relapsed or Refractory AML
The safety of REZLIDHIA 150 mg administered twice daily was evaluated in 153 adults with relapsed or refractory AML with an IDH1 mutation [see Clinical Studies (14.1)]. Among the 153 patients who received REZLIDHIA, 35% were exposed for at least 6 months and 21% were exposed for at least 1 year. The median duration of exposure to REZLIDHIA was 4.7 months (range: 0.1 to 34 months).
Serious adverse reactions occurred in 25% of patients who received REZLIDHIA. Serious adverse reactions in ≥5% included differentiation syndrome (9%) and transaminitis (6%). Fatal adverse reactions occurred in 1% of patients who received REZLIDHIA, due to differentiation syndrome.
Permanent discontinuation of REZLIDHIA due to an adverse reaction occurred in 8% of patients. Adverse reactions leading to permanent discontinuation in ≥1% of patients included transaminitis, differentiation syndrome, and gallbladder disorders.
Dosage interruptions of REZLIDHIA due to an adverse reaction occurred in 32% of patients. Adverse reactions which required dosage interruption in >5% of patients included transaminitis and differentiation syndrome.
Dose reductions of REZLIDHIA due to an adverse reaction occurred in 11% of patients. Adverse reactions which required dose reductions in ≥2% of patients included transaminitis.
The most common (≥20%) adverse reactions, including laboratory abnormalities, were aspartate aminotransferase increased, alanine aminotransferase increased, potassium decreased, sodium decreased, alkaline phosphatase increased, nausea, creatinine increased, fatigue/malaise, arthralgia, constipation, lymphocytes increased, bilirubin increased, leukocytosis, uric acid increased, dyspnea, pyrexia, rash, lipase increased, mucositis, diarrhea and transaminitis.
Table 2 summarizes the adverse reactions in the clinical trial for relapsed or refractory AML.
Table 2: Adverse Reactions (≥10%) in Patients with Relapsed or Refractory AML in the Clinical Trial - * Mucositis includes gingival hypertrophy, gingivitis, gingivitis ulcerative, oral disorder, colitis, mouth ulceration, stomatitis, tongue ulceration, oral pain, oropharyngeal pain, pharyngitis, proctalgia, and colitis ischemic.
- † Includes multiple similar adverse reaction terms.
- ‡ Arthralgia grouped term includes arthralgia, bone pain, back pain, neck pain, pain in extremity, arthritis, joint effusion, joint range of motion decreased, and joint swelling.
- § Includes fatal adverse reaction.
- ¶ Symptoms of differentiation syndrome in patients treated with REZLIDHIA included leukocytosis, dyspnea, pulmonary infiltrates/pleuropericardial effusion, kidney injury, fever, edema, pyrexia, and weight gain.
- # Dyspnea grouped term includes acute respiratory distress syndrome, dyspnea, dyspnea exertional, hypoxia, oxygen saturation decreased, respiratory distress, respiratory failure.
- Þ Transaminitis grouped term includes alanine aminotransferase increased, aspartate aminotransferase increased, hepatic enzyme increased, hypertransaminasemia, liver function test abnormal, liver function test increased, transaminases increased, hepatitis acute, and blood alkaline phosphatase increased.
Olutasidenib 150 mg BID
N=153Body System
Adverse ReactionAll Grades
(%)Grade 3 or 4
(%)Gastrointestinal Disorders Nausea 38 0 Constipation 26 0 Mucositis* 23 3 Diarrhea 20 1 Abdominal pain† 18 1 Vomiting 17 1 General Disorders and Administration Site Conditions Fatigue/malaise† 36 3 Pyrexia 24 1 Edema† 18 3 Musculoskeletal and Connective Tissue Disorders Arthralgia‡ 28 3 Blood System and Lymphatic Disorders Leukocytosis 25 9 Differentiation syndrome§'¶ 16 8 Respiratory, Thoracic and Mediastinal Disorders Dyspnea§'# 24 5 Cough† 17 1 Skin and subcutaneous tissue disorders Rash† 24 1 Investigations TransaminitisÞ 20 12 Metabolism and Nutrition Disorders Decreased appetite 16 2 Nervous System Disorders Headache 13 0 Vascular Disorders Hypertension† 10 5 Clinically relevant adverse reactions in <10% of patients who received REZLIDHIA include:
- Gallbladder disorders: biliary tract disorder, biliary colic, cholangitis, and cholestasis
- Electrocardiogram QT prolonged
Table 3 summarizes the laboratory abnormalities in the clinical trial for relapsed or refractory AML.
Table 3: Most Common (≥10%) New or Worsening Laboratory Abnormalities in Patients with Relapsed or Refractory AML in the Clinical Trial - * The denominator used to calculate the rate varied from 143 to 152 based on the number of patients with a baseline value and at least one post-treatment value.
Olutasidenib* Parameter All Grades
(%)Grade 3 or 4
(%)Aspartate aminotransferase increased 47 10 Alanine aminotransferase increased 46 13 Potassium decreased 46 9 Sodium decreased 42 7 Alkaline phosphatase increased 42 7 Creatinine increased 38 2 Lymphocytes increased 26 3 Bilirubin increased 26 2 Uric acid increased 25 3 Lipase increased 24 8 -
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on Olutasidenib
Strong or Moderate CYP3A4 Inducers
Avoid concomitant use of REZLIDHIA with strong or moderate CYP3A inducers.Olutasidenib is a CYP3A substrate. Concomitant use of REZLIDHIA with a strong CYP3A inducer decreases olutasidenib Cmax and AUC, which may reduce REZLIDHIA efficacy [see Clinical Pharmacology (12.3)].
Concomitant use of REZLIDHIA with a moderate CYP3A inducer may also decrease olutasidenib Cmax and AUC, which may also reduce REZLIDHIA efficacy, based on observations from concomitant use with a strong CYP3A inducer.
7.2 Effect of Olutasidenib on Other Drugs
Sensitive CYP3A Substrates
Avoid concomitant use of REZLIDHIA with sensitive CYP3A substrates unless otherwise instructed in the substrates prescribing information. If concomitant use is unavoidable, monitor patients for loss of therapeutic effect of these drugs.
Olutasidenib induces CYP3A. Concomitant use of REZLIDHIA may decrease plasma concentrations of sensitive CYP3A substrates, which may reduce the substrate’s efficacy [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on animal embryo-fetal toxicity studies, REZLIDHIA may cause fetal harm when administered to a pregnant woman. There are no available data on REZLIDHIA use in pregnant women to evaluate for a drug-associated risk.
In embryo-fetal development studies, oral olutasidenib resulted in embryo-fetal death and altered fetal growth when administered to pregnant rats and rabbits during the period of organogenesis at exposures up to 10 times and 0.7 times, respectively, the human exposure at the recommended daily dose (see Data). Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%, respectively.
Animal Data
Olutasidenib was administered twice daily via oral gavage at dose levels of 25, 125, or 250 mg/kg/dose (50, 250, or 500 mg/kg/day) to pregnant rats during organogenesis (gestation days 6-17). An increase in fetal supernumerary rib was observed at the high dose (10 times the AUC at the clinical dose of 150 mg BID). In a pilot study, administration of olutasidenib orally to pregnant rats during organogenesis resulted in an increase in post-implantation loss at doses of 250 and 450 mg/kg/day (9 and 10 times the AUC at the clinical dose of 150 mg BID).
Olutasidenib was administered twice daily via oral gavage at dose levels of 10, 20, or 40 mg/kg/dose (20, 40, or 80 mg/kg/day) to pregnant rabbits during the period of organogenesis (gestation days 7- 20). Maternal toxicity noted as reduced body weight gain occurred at 80 mg/kg/day. An increase in fetal supernumerary rib and increased post-implantation loss occurred at the high dose of 80 mg/kg/day (0.7 times the AUC at the clinical dose of 150 mg BID).
8.2 Lactation
Risk Summary
There are no data on the presence of olutasidenib or its metabolites in human milk, the effects on the breastfed child, or milk production. Because many drugs are excreted in human milk, and due to the potential for adverse reactions in a breastfed child, advise women not to breastfeed during treatment with REZLIDHIA and for 2 weeks after the last dose.
8.4 Pediatric Use
The safety and effectiveness of REZLIDHIA have not been established in pediatric patients.
8.5 Geriatric Use
Among the 153 patients with relapsed or refractory AML with an IDH1 mutation treated with REZLIDHIA, 116 (76%) were 65 years of age or older and 48 (31%) were 75 years or older. No overall differences in effectiveness were observed between patients 65 years and older and younger patients. Compared to patients younger than 65 years of age, an increase in incidence of hepatotoxicity and hypertension was observed in patients ≥65 years of age.
8.6 Renal Impairment
No dosage modification is recommended for patients with mild to moderate renal impairment (creatinine clearance [CLcr] 30 to <90 mL/min, as estimated by Cockcroft-Gault). The recommended dosage of REZLIDHIA has not been established in patients with severe renal impairment (CLcr 15 to 29 mL/min as estimated by Cockcroft-Gault), kidney failure (CLcr <15 mL/min, as estimated by Cockcroft-Gault), and patients on dialysis [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
No dosage modification is recommended for patients with mild (total bilirubin ≤ULN and any AST >ULN or total bilirubin >1 to 1.5 times ULN and any AST) or moderate (total bilirubin >1.5 to 3 times ULN and any AST) hepatic impairment [see Clinical Pharmacology (12.3)]. In patients with mild or moderate hepatic impairment, closely monitor for increased probability of differentiation syndrome [see Dosage and Administration (2.3) and Warnings and Precautions (5.1)]. The recommended dosage of REZLIDHIA has not been established in patients with severe hepatic impairment (total bilirubin >3 times ULN with any AST) [see Clinical Pharmacology (12.3)].
-
11 DESCRIPTION
Olutasidenib is an isocitrate dehydrogenase-1 (IDH1) inhibitor. The chemical name is (S)-5-((1-(6-chloro-2-oxo-1,2-dihydroquinolin-3-yl)ethyl)amino)-1-methyl-6-oxo-1,6-dihydropyridine-2-carbonitrile. The chemical structure is:
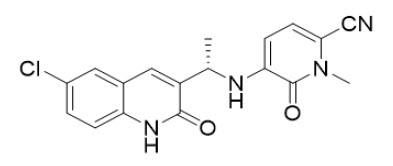
The molecular formula is C18H15ClN4O2 and the molecular weight is 354.79 g/mol. Olutasidenib is a white to off-white to brown powder that is practically insoluble in aqueous solutions between pH 1.2 and 7.4.
REZLIDHIA (olutasidenib) is available as hard gelatin capsules for oral administration. Each capsule contains 150 mg olutasidenib and the following ingredients: croscarmellose sodium, magnesium stearate and microcrystalline cellulose. The capsule shell contains gelatin and titanium dioxide. Each capsule is printed with black ink containing ferrosoferric oxide, propylene glycol, and shellac.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Olutasidenib is a small-molecule inhibitor of mutated isocitrate dehydrogenase-1 (IDH1). In patients with AML, susceptible IDH1 mutations are defined as those leading to increased levels of 2-hydroxyglutarate (2-HG) in the leukemia cells and where efficacy is predicted by 1) clinically meaningful remissions with the recommended dose of olutasidenib and/or 2) inhibition of mutant IDH1 enzymatic activity at concentrations of olutasidenib sustainable at the recommended dosage according to validated methods. The most common of such mutations in patients with AML are R132H and R132C substitutions.
In vitro, olutasidenib inhibited mutated IDH1 R132H, R132L, R132S, R132G, and R132C proteins; wild-type IDH1 or mutated IDH2 proteins were not inhibited. Olutasidenib inhibition of mutant IDH1 led to decreased 2-HG levels in vitro and in in vivo xenograft models.
12.2 Pharmacodynamics
The mean [% coefficient of variation (%CV)] reduction in 2-HG plasma concentration was 59.1% (122%) by pre-dose Cycle 2 and was sustained throughout the treatment period in patients with AML and IDH1 mutations following the approved recommended olutasidenib dosage.
Increased olutasidenib exposure was correlated with the increased probability of differentiation syndrome and Grade ≥3 hepatotoxicity in patients with AML following the approved recommended olutasidenib dosage.
Cardiac Electrophysiology
The largest mean increase in QTc interval was 6.2 msec (upper 90% confidence interval = 9.7 msec) in 33 patients with advanced hematologic malignancies with an IDH1 mutation following a single dose and multiple doses of the approved recommended olutasidenib dosage under fasted conditions. This increase in the QTc interval was concentration dependent.
Increased QT prolongation is expected with increased exposures of olutasidenib under a fed condition compared to that under fasting conditions [see Clinical Pharmacology (12.3)]. The clinical impact of this increase could not be determined because QTc intervals were not evaluated at higher olutasidenib exposures.
12.3 Pharmacokinetics
The pharmacokinetics of olutasidenib have been characterized in patients with AML following the approved recommended dosage, unless otherwise specified.
The mean (%CV) olutasidenib steady-state daily area under the plasma drug concentration over time curve (AUC0-12-h,ss) is 43050 (34.0%) ng·h/mL and steady-state maximum plasma concentration (Cmax,ss) is 3573 (45.6%) ng/mL following the approved recommended dosage.
Olutasidenib Cmax and AUC increase less-than proportionally over a dosage range from 100 mg to 300 mg (0.33 to 1 time the recommended total daily dose); however, this finding should not affect the recommended dosage of REZLIDHIA. Olutasidenib accumulation ratios ranging from 7.7 and 9.5 were observed following the approved recommended dosage. Steady-state plasma levels are reached within 14 days.
Absorption
The median (min, max) time to maximum concentration (tmax) of olutasidenib is approximately 4 (1, 8) hours following a single oral dose of 150 mg.
Effect of Food
The mean (CV%) of olutasidenib Cmax increased by 191% (20.6%) and AUCinf increased by 83% (18.3%) following administration of a single 150 mg dose of olutasidenib with a high-fat meal (approximately 800 to 1,000 calories, with approximately 50% of total caloric content of the meal from fat) in healthy subjects.
Distribution
The mean (CV%) apparent volume of distribution of olutasidenib is 319 (28.1%) L. The plasma protein binding of olutasidenib is approximately 93%.
Elimination
The mean (CV%) half-life (t1⁄2) of olutasidenib is approximately 67 (51.2%) hours and the mean (CV%) apparent oral clearance (CL/F) of olutasidenib is 4 (60.5%) L/h.
Metabolism
Olutasidenib metabolism involves N-dealkylation, demethylation, oxidative deamination followed by oxidation, mono-oxidation with subsequent glucuronidation. Olutasidenib is primarily (90%) metabolized by cytochrome P450(CYP)3A4, with minor contributions from CYP2C8, CYP2C9, CYP1A2, and CYP2C19.
Excretion
Following a single oral radiolabeled olutasidenib dose of 150 mg to healthy subjects, approximately 75% of olutasidenib was recovered in feces (35% unchanged) and 17% in the urine (1% unchanged).
Specific Populations
No clinically significant differences in the pharmacokinetics of olutasidenib were observed based on age (28 to 90 years), sex, body weight (36 to 145 kg), mild to moderate renal impairment (creatinine clearance [CLcr] 30 to <90mL/min as estimated by Cockcroft-Gault), or mild (total bilirubin ≤ULN and any AST >ULN or total bilirubin >1 to 1.5 times ULN and any AST) or moderate (total bilirubin >1.5 to 3 times ULN and any AST) hepatic impairment.
The effect of severe renal impairment (CLcr 15 to 29 mL/min, as estimated by Cockcroft-Gault), kidney failure (CLcr <15 mL/min, as estimated by Cockcroft-Gault), patients on dialysis, and patients with severe hepatic impairment (total bilirubin >3 x ULN with any AST) on olutasidenib pharmacokinetics is unknown or not fully characterized.
Drug Interaction Studies
Clinical Studies
Strong CYP3A and P-glycoprotein (P-gp) Inhibitors: No clinically significant differences in olutasidenib pharmacokinetics were observed when used concomitantly with multiple doses of a strong CYP3A and P-gp inhibitor (itraconazole).
Strong CYP3A4 Inducers: Olutasidenib Cmax decreased by 43% and AUC by 80% when used concomitantly with multiple doses of a strong CYP3A inducer (rifampin).
In vitro Studies
CYP Enzymes: Olutasidenib induces CYP3A4, CYP2B6, CYP1A2, CYP2C8 and CYP2C9.
Olutasidenib does not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A4/5.
Transporter Systems: Olutasidenib is not a substrate of BCRP, BSEP, MRP2, MRP3, or MRP4.
Ivosidenib does not inhibit BCRP, OATP1B1, OATP1B3, OAT1, and OCT2 at clinically relevant concentrations. Ivosidenib is an inhibitor of OAT3 and P-gp.
Olutasidenib is an inhibitor of P-gp, BCRP, OATP1B1, OATP1B3, OAT3, OCT2, MATE1, and MATE2K. Olutasidenib does not inhibit BSEP, MRP2, MRP3, MRP4, or OAT1.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with olutasidenib. Olutasidenib was not genotoxic in the in vitro bacterial reverse mutation (Ames), in vitro human lymphocyte micronucleus, and in vivo rat bone marrow micronucleus assays. Fertility studies in animals have not been conducted with olutasidenib.
-
14 CLINICAL STUDIES
14.1 Acute Myeloid Leukemia
The efficacy of REZLIDHIA was evaluated in an open-label, single-arm, multicenter clinical trial (Study 2102-HEM-101, NCT02719574) in 147 adult patients with relapsed or refractory AML with an IDH1 mutation.
IDH1 mutations in blood or bone marrow were confirmed retrospectively using the Abbott RealTime™ IDH1 Assay. REZLIDHIA was given orally at a dose of 150 mg twice daily until disease progression, development of unacceptable toxicity, or hematopoietic stem cell transplantation. Sixteen of the 147 patients (11%) underwent stem cell transplantation following REZLIDHIA treatment.
The baseline demographic and disease characteristics are shown in Table 4.
Table 4: Baseline Demographic and Disease Characteristics in Patients with Relapsed or Refractory AML (Study 2102-HEM-101) - * Using central IDH1 assay testing results.
- † Cytogenetic risk categorization was investigator reported by NCCN or ELN guidelines.
- ‡ May be first or subsequent relapse.
- § Transfusion-Dependent at Baseline is defined as receiving a transfusion within 8 weeks prior to first dose of olutasidenib or noting transfusion dependence prior to coming on study.
Demographic and Disease Characteristics REZLIDHIA (150 mg twice daily)
N=147Demographics Age (Years) Median (Min, Max) 71 (32, 87) Age Categories, n (%) <65 years 37 (25) ≥65 years to <75 years 65 (44) ≥75 years 45 (31) Sex, n (%) Male 74 (50) Female 73 (50) Race, n (%) White 67 (46) Black or African American 5 (3) Asian 5 (3) Native Hawaiian/Other Pacific Islander 0 (0) Other/Not provided 70 (48) Disease Characteristics ECOG PS, n (%) 0 45 (31) 1 76 (52) 2 23 (16) IDH1 Mutation, n (%)* R132C 85 (58) R132H 35 (24) R132G 12 (8) R132S 11 (7) R132L 4 (3) Type of AML, n (%) De novo AML 97 (66) Secondary AML 50 (34) Cytogenetic Risk Status†, n (%) Favorable 6 (4) Intermediate 107 (73) Poor 25 (17) Unknown 9 (6) Relapsed/Refractory Patient Category Primary Refractory 46 (31) Untreated Relapse‡ 81 (55) Refractory Relapse‡ 20 (14) Relapse Number 0 46 (31) 1 87 (59) 2 11 (8) ≥3 3 (2) Prior Stem Cell Transplantation for AML, n (%) 17 (12) Transfusion Dependent at Baseline§, n (%) 86 (59) Median Number of Prior Therapies (Min, Max) 2 (1,7) Efficacy was established on the basis of the rate of complete remission (CR) plus complete remission with partial hematologic recovery (CRh), the duration of CR+CRh, and the rate of conversion from transfusion dependence to transfusion independence. The efficacy results are shown in Table 5. The median follow-up was 10.2 months (range: 0.2 to 38.1 months) and median treatment duration was 4.7 months (range: 0.1 to 26.0 months).
Table 5: Efficacy Results in Patients with Relapsed or Refractory AML (Study 2102-HEM-101) - * CR (complete remission) was defined as <5% blasts in the bone marrow, no blasts with Auer rods, no extramedullary disease, and full recovery of peripheral blood counts (platelets >100,000/microliter and absolute neutrophil counts [ANC] >1,000/microliter); CRh (complete remission with partial hematologic recovery) was defined as <5% blasts in the bone marrow, no evidence of disease, and partial recovery of peripheral blood counts (platelets >50,000/microliter and ANC >500/microliter).
- † CR+CRh rate was consistent across all baseline demographic and baseline disease characteristic subgroups with the exception of IDH1 R132H mutation (CR+CRh 17%).
- ‡ Duration of response is defined as the time from the date of the first response to the date of the relapse or death. Patients who did not relapse were censored at the date of last response assessment. + indicates censored observation.
Endpoint REZLIDHIA (150 mg twice daily) N=147 CR+CRh*,† n (%) 51 (35) 95% CI (27, 43) Median DOCR+CRh‡ (months) 25.9 95% CI (13.5, NR) CR* n (%) 47 (32) 95% CI (25, 40) Median DOCR‡ (months) 28.1 95% CI (13.8, NR) CRh* n (%) 4 (2.7) 95% CI (0.7, 6.8) Observed DOCRh‡ (months) 1.8, 5.6, 13.5, 28.5+ CI: confidence interval; NR = not reached Of the patients who achieved a CR or CRh, the median time to CR or CRh was 1.9 months (range: 0.9 to 5.6 months). All patients that achieved a best response of CR or CRh did so within 5.6 months of initiating REZLIDHIA.
Overall, among the 86 patients who were dependent on red blood cell (RBC) and/or platelet transfusions at baseline, 29 (34%) became independent of RBC and platelet transfusions during any 56-day post-baseline period. Of the 61 patients who were independent of both RBC and platelet transfusions at baseline, 39 (64%) remained transfusion independent during any 56-day post- baseline period.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Capsule Strength Description Package Configuration NDC Number 150 mg White hard gelatin capsules with black ink print "OLU 150" White high-density polyethylene (HDPE) bottle with child-resistant closure 71332-005-01 Each bottle contains 30 capsules Storage
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F)
[see USP Controlled Room Temperature]. -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Differentiation Syndrome
Advise patients of the risks of developing differentiation syndrome as early as 1 day after start of therapy and up to 18 months on treatment. Ask patients to immediately report any symptoms suggestive of differentiation syndrome, such as fever, cough or difficulty breathing, decreased urinary output, low blood pressure, weight gain, or swelling of their arms or legs, to their healthcare provider for further evaluation [see Warnings and Precautions (5.1)].
Hepatotoxicity
Advise patients of the potential for hepatic effects and to immediately report any associated signs and symptoms such as right upper abdominal discomfort, dark urine, jaundice, anorexia, or fatigue to their healthcare provider for further evaluation [see Warnings and Precautions (5.2)].
Gastrointestinal Adverse Reactions
Advise patients on the risks of experiencing gastrointestinal reactions such as nausea, constipation, diarrhea, vomiting, abdominal pain, and mucositis. Ask patients to report these events to their healthcare provider and advise patients how to manage them [see Adverse Reactions (6.1)].
Lactation
Advise women not to breastfeed during treatment with REZLIDHIA and for 2 weeks after the last dose [see Use in Specific Populations (8.2)].
Dosing and Storage Instructions
- Advise patients to swallow capsules whole. Do not break, open, or chew the capsules.
- Advise patients to take REZLIDHIA on an empty stomach (at least 1 hour before or 2 hours after a meal).
- Advise patients that if a dose of REZLIDHIA is vomited, do not administer a replacement dose; wait until the next scheduled dose is due.
- If a dose of REZLIDHIA is missed or not taken at the usual time, instruct patients to take the dose as soon as possible unless the next dose is due within 8 hours. Patients can return to the normal schedule the following day.
- Store REZLIDHIA at room temperature from 20°C to 25°C (68°F to 77°F).
Manufactured by Catalent Greenville, Inc. 1240 Sugg Pkwy, Greenville, NC 27834
Manufactured for Rigel Pharmaceuticals, Inc. South San Francisco, CA 94080REZLIDHIA® is a trademark of Rigel Pharmaceuticals, Inc.
For more information go to www.REZLIDHIA.com or call 1-800-983-1329.04/2024
-
MEDICATION GUIDE
MEDICATION GUIDE
REZLIDHIA® (REZ-LID-EE-AH)
(olutasidenib) capsulesWhat is the most important information I should know about REZLIDHIA?
REZLIDHIA may cause serious side effects including:
-
Differentiation Syndrome. Differentiation syndrome is a condition that affects your blood cells and may be life- threatening or lead to death. Differentiation syndrome in adults with acute myeloid leukemia (AML) has occurred as early as 1 day and up to 18 months after starting REZLIDHIA. Call your healthcare provider or go to the nearest hospital emergency room right away if you develop any of the following symptoms of differentiation syndrome during treatment with REZLIDHIA:
- fever
- cough
- trouble breathing
- decreased urination
- dizziness or lightheadedness
- weight gain
- swelling of your arms or legs
If you develop signs and symptoms of differentiation syndrome, your healthcare provider may treat you with a corticosteroid medicine or a medicine called hydroxyurea and may monitor you in the hospital.
See "What are the possible side effects of REZLIDHIA?" for more information about side effects.
What is REZLIDHIA?
REZLIDHIA is a prescription medicine used to treat adults with acute myeloid leukemia (AML) with an isocitrate dehydrogenase-1 (IDH1) mutation when the disease has come back or has not improved after previous treatment(s).
Your healthcare provider will perform a test to make sure that REZLIDHIA is right for you.
It is not known if REZLIDHIA is safe and effective in children.
Before taking REZLIDHIA, tell your healthcare provider about all of your medical conditions, including if you:
- have liver problems.
- are pregnant or plan to become pregnant. REZLIDHIA may harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if REZLIDHIA passes into your breast milk. Do not breastfeed during your treatment with REZLIDHIA and for 2 weeks after your last dose of REZLIDHIA.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I take REZLIDHIA?
- Take REZLIDHIA exactly as your healthcare provider tells you to.
- Do not change your dose or stop taking REZLIDHIA without talking to your healthcare provider.
- Take REZLIDHIA 2 times a day (about 12 hours apart) around the same times each day.
- Take REZLIDHIA on an empty stomach, at least 1 hour before or 2 hours after a meal.
- Swallow REZLIDHIA capsules whole. Do not break, open or chew the capsules.
- If you vomit after taking a dose of REZLIDHIA, do not take another dose. Take your next dose at your usual time.
- If you miss a dose of REZLIDHIA or did not take it at the usual time, take your dose as soon as possible and at least 8 hours before your next dose. Return to your normal schedule the following day. Do not take 2 doses of REZLIDHIA within 8 hours.
What are the possible side effects of REZLIDHIA?
REZLIDHIA may cause serious side effects, including:
- See “What is the most important information I should know about REZLIDHIA?”
-
Liver problems. Changes in liver function tests are common during treatment with REZLIDHIA and can be serious. Your healthcare provider will do blood tests to check your liver function before and during treatment with REZLIDHIA. Tell your healthcare provider right away if you develop any of the following symptoms of liver problems during treatment with REZLIDHIA:
- yellowing of your skin or the white part of your eyes (jaundice)
- dark “tea-colored” urine
- loss of appetite
- pain on the upper right side of your stomach area
- feeling very tired or weak
The most common side effects of REZLIDHIA in adults with AML include:
- abnormal liver function tests
- nausea
- feeling tired or unwell
- joint pain
- constipation
- shortness of breath
- fever
- rash
- mouth sores
- diarrhea
- changes in certain blood tests
Tell your healthcare provider if you have any nausea, constipation, diarrhea, vomiting, stomach pain or mouth sores.
Your healthcare provider will do blood tests before you start and during treatment with REZLIDHIA. Your healthcare provider may decrease, temporarily hold, or permanently stop your treatment with REZLIDHIA if you develop certain side effects.
These are not all the possible side effects of REZLIDHIA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store REZLIDHIA?
Store REZLIDHIA at room temperature from 68°F to 77°F (20°C to 25°C).
Keep REZLIDHIA and all medicines out of the reach of children.
General information about the safe and effective use of REZLIDHIA
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not take REZLIDHIA for conditions for which it was not prescribed. Do not give REZLIDHIA to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about REZLIDHIA that is written for healthcare professionals.
What are the ingredients in REZLIDHIA?
Active ingredient: olutasidenib
Inactive ingredients:
croscarmellose sodium, magnesium stearate, and microcrystalline cellulose. The capsule shell contains gelatin and titanium dioxide. Each capsule is printed with black ink containing ferrosoferric oxide, propylene glycol and shellac.
Manufactured by Catalent Greenville, Inc. 1240 Sugg Pkwy, Greenville, NC 27834
Manufactured for Rigel Pharmaceuticals, Inc. South San Francisco, CA 94080
REZLIDHIA® is a trademark of Rigel Pharmaceuticals, Inc.
For more information go to www.REZLIDHIA.com or call 1-800-983-1329.This Medication Guide has been approved by the U.S. Food and Drug Administration.
Issued: 04/2024
-
Differentiation Syndrome. Differentiation syndrome is a condition that affects your blood cells and may be life- threatening or lead to death. Differentiation syndrome in adults with acute myeloid leukemia (AML) has occurred as early as 1 day and up to 18 months after starting REZLIDHIA. Call your healthcare provider or go to the nearest hospital emergency room right away if you develop any of the following symptoms of differentiation syndrome during treatment with REZLIDHIA:
-
PRINCIPAL DISPLAY PANEL - REZLIDHIA - NDC: 71332-005-01 - 150 mg Capsule 30-Count Carton Label
NDC: 71332-005-01
Rx only
REZLIDHIA®
olutasidenib capsules
150 mg
Dispense the enclosed Medication Guide to each patient.
30 capsules

-
INGREDIENTS AND APPEARANCE
REZLIDHIA
olutasidenib capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 71332-005 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OLUTASIDENIB (UNII: 0T4IMT8S5Z) (OLUTASIDENIB - UNII:0T4IMT8S5Z) OLUTASIDENIB 150 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) Product Characteristics Color WHITE Score no score Shape CAPSULE Size 23mm Flavor Imprint Code OLU;150 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71332-005-01 30 in 1 BOTTLE; Type 0: Not a Combination Product 12/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215814 12/01/2022 Labeler - Rigel Pharmaceuticals, Inc. (967965468) Establishment Name Address ID/FEI Business Operations Catalent Greenville, Inc. 118812386 MANUFACTURE(71332-005)
Trademark Results [REZLIDHIA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 REZLIDHIA 97683809 not registered Live/Pending |
Rigel Pharmaceuticals, Inc. 2022-11-18 |
 REZLIDHIA 88603967 not registered Live/Pending |
Forma Therapeutics, Inc. 2019-09-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.