ONYDA XR- clonidine hydrochloride suspension, extended release
ONYDA XR by
Drug Labeling and Warnings
ONYDA XR by is a Prescription medication manufactured, distributed, or labeled by NextWave Pharmaceuticals, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ONYDATM XR safely and effectively. See full prescribing information for ONYDATM XR.
ONYDATM XR (clonidine hydrochloride) extended-release oral suspension
Initial U.S. Approval: 1974RECENT MAJOR CHANGES
Warnings and Precautions (5.3) 04/2025
INDICATIONS AND USAGE
ONYDA XR is a centrally acting alpha2-adrenergic agonist indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) as monotherapy or as adjunctive therapy to central nervous system (CNS) stimulant medications in pediatric patients 6 years of age and older. (1)
DOSAGE AND ADMINISTRATION
- Starting dosage is 0.1 mg of ONYDA XR orally once daily at bedtime with or without food. Dosage may be increased in increments of 0.1 mg per day at weekly intervals. Maximum recommended dosage is 0.4 mg once daily at bedtime. (2.1)
- Do not substitute ONYDA XR for other clonidine products on a mg-per-mg basis because of differing pharmacokinetic profiles. (2.3)
- When discontinuing, taper the dose in decrements of no more than 0.1 mg every 3 to 7 days to avoid rebound hypertension. (2.4)
DOSAGE FORMS AND STRENGTHS
Extended-release oral suspension: 0.1 mg clonidine hydrochloride per mL (3)
CONTRAINDICATIONS
History of a hypersensitivity reaction to clonidine. Reactions have included generalized rash, urticaria, angioedema. (4)
WARNINGS AND PRECAUTIONS
- Hypotension/bradycardia: Titrate slowly and monitor vital signs frequently in patients at risk for hypotension, heart block, bradycardia, syncope, cardiovascular disease, vascular disease, cerebrovascular disease, or chronic renal failure. Measure heart rate and blood pressure prior to initiation of therapy, following dose increases, and periodically while on therapy. Avoid concomitant use of drugs with additive effects unless clinically indicated. Advise patients to avoid becoming dehydrated or overheated. (5.1)
- Somnolence/Sedation: Has been observed with clonidine. Consider the potential for additive sedative effects with CNS depressant drugs. Caution patients against operating heavy equipment or driving until they know how they respond to ONYDA XR (5.2)
- Cardiac Conduction Abnormalities: May worsen sinus node dysfunction and atrioventricular (AV) block, especially in patients taking other sympatholytic drugs. Titrate slowly and monitor vital signs frequently. (5.5)
ADVERSE REACTIONS
Most common adverse reactions (incidence at least 5% and twice the rate of placebo) as monotherapy in ADHD: somnolence, fatigue, irritability, nightmare, insomnia, constipation, dry mouth. (6.1)
Most common adverse reactions (incidence at least 5% and twice the rate of placebo) as adjunct therapy to psychostimulant in ADHD: somnolence, fatigue, decreased appetite, dizziness. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Tris Pharma, Inc. at (732) 940-0358 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- CNS Depressants: Clonidine may potentiate the CNS-depressive effects of alcohol, barbiturates or other sedating drugs. (7)
- Tricyclic Antidepressants: May reduce the hypotensive effect of clonidine. (7)
- Drugs Known to Affect Sinus Node Function or AV Nodal Conduction: Avoid use of ONYDA XR with agents known to affect sinus node function or AV nodal conduction (e.g., digitalis, calcium channel blockers and beta-blockers) due to a potential for additive effects such as bradycardia and AV block. (7)
- Antihypertensive drugs: Use caution when coadministered with ONYDA XR. (7)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 4/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
RECENT MAJOR CHANGES
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Administration Instructions
2.3 Switching from Other Clonidine Products
2.4 Discontinuation
2.5 Missed Doses
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypotension/Bradycardia
5.2 Sedation and Somnolence
5.3 Rebound Hypertension
5.4 Allergic Reactions
5.5 Cardiac Conduction Abnormalities
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis and Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
ONYDA XR is indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) as monotherapy and as adjunctive therapy to central nervous system (CNS) stimulant medications in pediatric patients 6 years of age and older [see Clinical Studies (14)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The starting dosage of ONYDA XR is 0.1 mg orally once daily at bedtime with or without food [see Clinical Pharmacology (12.3)]. Titrate the dose of ONYDA XR in increments of 0.1 mg per day at weekly intervals depending on clinical response up to the maximum recommended dosage of 0.4 mg once daily at bedtime.
Doses of ONYDA XR higher than 0.4 mg once daily were not evaluated in clinical trials for ADHD and are not recommended.
When ONYDA XR is added to a CNS stimulant, adjust the dose of the CNS stimulant depending on the clinical response to ONYDA XR.
2.2 Administration Instructions
Instruct patients to read the “Instructions for Use” for complete administration instructions.
- Use the oral dosing dispenser and bottle adapter provided with ONYDA XR.
- Ensure that the bottle adapter is firmly inserted into the bottle before first use and keep the adapter in place for the duration of the usage of the bottle.
- Gently shake ONYDA XR with a smooth up and down motion (to avoid foaming) for at least 10 seconds before each administration.
- For the bottles of 30 mL and 60 mL, discard any unused ONYDA XR 30 days after first opening the bottle.
- For the 120 mL bottle, discard any unused ONYDA XR 60 days after first opening the bottle.
2.3 Switching from Other Clonidine Products
For patients switching from another clonidine product, discontinue that treatment, and titrate with ONYDA XR using the titration schedule [see Dosage and Administration (2.1)]. Do not substitute for other clonidine products on a milligram-per-milligram basis because of differing pharmacokinetic profiles [see Clinical Pharmacology (12.3)].
2.4 Discontinuation
When discontinuing ONYDA XR, taper the total daily dose in decrements of no more than 0.1 mg every 3 to 7 days to avoid rebound hypertension [see Warnings and Precautions (5.3)].
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypotension/Bradycardia
Treatment with ONYDA XR can cause dose-related decreases in blood pressure and heart rate [see Adverse Reactions (6.1)]. Measure heart rate and blood pressure prior to initiation of therapy, following dose increases, and periodically while on therapy. Titrate ONYDA XR slowly in patients with a history of hypotension, and those with underlying conditions that may be worsened by hypotension and bradycardia; e.g., heart block, bradycardia, cardiovascular disease, vascular disease, cerebrovascular disease, or chronic renal failure. In patients who have a history of syncope or may have a condition that predisposes them to syncope, such as hypotension, orthostatic hypotension, bradycardia, or dehydration, advise patients to avoid becoming dehydrated or overheated. Monitor blood pressure and heart rate, and adjust dosages accordingly in patients treated concomitantly with antihypertensives or other drugs that can reduce blood pressure or heart rate or increase the risk of syncope [see Drug Interactions (7)].
5.2 Sedation and Somnolence
Somnolence and sedation were commonly reported adverse reactions in clinical studies with clonidine hydrochloride extended-release tablets. In patients that completed 5 weeks of therapy in a controlled, fixed dose pediatric monotherapy study, 31% of patients treated with 0.4 mg/day and 38% treated with 0.2 mg/day versus 4% of placebo treated patients reported somnolence as an adverse reaction. In patients that completed 5 weeks of therapy in a controlled flexible dose pediatric adjunctive to stimulants study, 19% of patients treated with clonidine hydrochloride extended-release tablets plus a stimulant versus 7% treated with placebo plus a stimulant reported somnolence.
Before using ONYDA XR with other centrally active depressants (such as phenothiazines, barbiturates, or benzodiazepines), consider the potential for additive sedative effects [see Drug Interactions (7)]. Caution patients against operating heavy equipment or driving until they know how they respond to treatment with ONYDA XR. Advise patients to avoid use with alcohol.
5.3 Rebound Hypertension
Abrupt discontinuation of ONYDA XR can cause rebound hypertension. In adults with hypertension, sudden cessation of clonidine extended-release formulation treatment in the 0.2 to 0.6 mg per day range resulted in reports of headache, tachycardia, nausea, flushing, warm feeling, brief lightheadedness, tightness in chest, and anxiety. In adults with hypertension, sudden cessation of treatment with immediate-release clonidine has, in some cases, resulted in symptoms such as nervousness, agitation, headache, and tremor accompanied or followed by a rapid rise in blood pressure and elevated catecholamine concentrations in the plasma. Pediatric patients with gastrointestinal illnesses that lead to vomiting may result in missed doses of ONYDA XR, increasing the risk for rebound hypertension.
No studies evaluating abrupt discontinuation of clonidine hydrochloride extended-release tablets in pediatric patients with ADHD have been conducted; however, to minimize the risk of rebound hypertension, gradually reduce the dose of ONYDA XR in decrements of no more than 0.1 mg every 3 to 7 days. Patients should be instructed not to discontinue ONYDA XR therapy without consulting their physician due to the potential risk of withdrawal effects.
5.4 Allergic Reactions
In patients who have developed localized contact sensitization to clonidine transdermal system, continuation of clonidine transdermal system or use of oral ONYDA XR therapy may be associated with the development of a generalized skin rash.
In patients who develop an allergic reaction from clonidine transdermal system, use of ONYDA XR may also elicit an allergic reaction (including generalized rash, urticaria, or angioedema).
5.5 Cardiac Conduction Abnormalities
The sympatholytic action of clonidine may worsen sinus node dysfunction and atrioventricular (AV) block, especially in patients taking other sympatholytic drugs. There have been post-marketing reports of patients with conduction abnormalities and/or taking other sympatholytic drugs who developed severe bradycardia requiring intravenous (IV) atropine, IV isoproterenol, and temporary cardiac pacing while taking clonidine. Titrate ONYDA XR slowly and monitor vital signs frequently in patients with cardiac conduction abnormalities or patients concomitantly treated with other sympatholytic drugs.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described in greater detail elsewhere in labeling:
- Hypotension/bradycardia [see Warnings and Precautions (5.1)]
- Sedation and somnolence [see Warnings and Precautions (5.2)]
- Rebound hypertension [see Warnings and Precautions (5.3)]
- Allergic reactions [see Warnings and Precautions (5.4)]
- Cardiac Conduction Abnormalities [see Warnings and Precautions (5.5)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of ONYDA XR for the treatment of ADHD in pediatric patients 6 years and older is based upon adequate and well-controlled studies of clonidine hydrochloride extended-release tablets (referred to as “clonidine hydrochloride extended-release” in this section). The safety results of these adequate and well-controlled studies of clonidine hydrochloride extended-release tablets are presented below.
Two clonidine hydrochloride extended-release ADHD clinical studies (Study 1 and Study 2) evaluated 256 patients in two 8-week placebo-controlled studies.
A third clonidine hydrochloride extended-release ADHD clinical study (Study 3) evaluated 135 pediatric patients 6 to 17 years of age in a 40-week placebo-controlled randomized‑withdrawal study.
Study 1: Fixed-dose clonidine hydrochloride extended-release Monotherapy
Study 1 was a short-term, multi-center, randomized, double-blind, placebo-controlled study of two fixed doses (0.2 mg/day or 0.4 mg/day) of clonidine hydrochloride extended-release in pediatric patients 6 to 17 years of age who met DSM-IV criteria for ADHD hyperactive or combined inattentive/hyperactive subtypes.
Most Common Adverse Reactions (incidence of ≥ 5% and at least twice the rate of placebo): somnolence, fatigue, irritability, insomnia, nightmare, constipation, dry mouth.
Adverse Reactions Leading to Discontinuation of clonidine hydrochloride extended-release: Five patients (7%) in the low dose group (0.2 mg), 15 patients (20%) in the high dose group (0.4 mg), and 1 patient in the placebo group (1%) reported adverse reactions that led to discontinuation. The most common adverse reactions that led to discontinuation were somnolence and fatigue.
Commonly observed adverse reactions (incidence of ≥2% in either active treatment group and greater than the rate on placebo) during the treatment period are listed in Table 1.
Table 1: Common Adverse Reactions Occurring in ≥2%of Patients Treated with Clonidine Hydrochloride Extended-Release Tablets and Greater than the Rate of Placebo in the Fixed-Dose Monotherapy Trial -Treatment Period (Study 1)
Preferred Term
Clonidine hydrochloride extended-release tablets
0.2 mg/day
N=76
(%)
Clonidine hydrochloride extended-release tablets
0.4 mg/day
N=78
(%)
Placebo N=76
(%)
PSYCHIATRIC DISORDERS
Somnolence*
Nightmare
Emotional Disorder
Aggression
Tearfulness
Enuresis
Sleep Terror
Poor Quality Sleep
38
4
4
3
1
0
3
0
31
9
4
1
3
4
0
3
4
0
1
0
0
0
0
1
NERVOUS SYSTEM DISORDERS
Headache
Insomnia
Tremor
Abnormal Sleep-Related Event
20
5
1
3
13
6
4
1
16
1
0
0
GASTRO-INTESTINAL DISORDERS
Upper Abdominal Pain
Nausea
Constipation
Dry Mouth
15
4
1
0
10
5
6
5
12
3
0
1
GENERAL DISORDERS
Fatigue†
Irritability
16
9
13
5
1
4
CARDIAC DISORDERS
Dizziness
Bradycardia
7
0
3
4
5
0
INVESTIGATIONS
Increased Heart Rate
0
3
0
METABOLISM AND NUTRITION DISORDERS
Decreased Appetite
3
4
4
* Somnolence includes the terms "somnolence" and "sedation".
† Fatigue includes the terms "fatigue" and "lethargy".
Commonly observed adverse reactions (incidence of ≥2% in either active treatment group and greater than the rate on placebo) during the taper period are listed in Table 2.
Table 2: Common Adverse Reactions Occurring in ≥2% of Patients Treated with Clonidine Hydrochloride Extended-Release Tablets and Greater than the Rate of Placebo in the Fixed-Dose Monotherapy Trial -Taper Period* (Study 1)
Preferred Term
Clonidine hydrochloride extended-release tablets
0.2 mg/day
N=76
(%)
Clonidine hydrochloride extended-release tablets
0.4 mg/day
N=78
(%)
Placebo
N=76
(%)
Abdominal Pain Upper
0
6
3
Headache
5
2
3
Gastrointestinal Viral
0
5
0
Somnolence
2
3
0
Heart Rate Increased
0
3
0
Otitis Media Acute
3
0
0
* Taper Period: 0.2 mg dose, week 8; 0.4 mg dose, weeks 6-8; Placebo dose, weeks 6-8
Study 2: Flexible-dose clonidine hydrochloride extended-release as Adjunctive Therapy to Psychostimulants
Study 2 was a short-term, randomized, double-blind, placebo-controlled study of a flexible dose of clonidine hydrochloride extended-release as adjunctive therapy to a psychostimulant in pediatric patients 6 to 17 years of age who met DSM-IV criteria for ADHD hyperactive or combined inattentive/hyperactive subtypes, during which clonidine hydrochloride extended‑release was initiated at 0.1 mg/day and titrated up to 0.4 mg/day over a 3-week period. Most clonidine hydrochloride extended-release treated patients (75.5%) were escalated to the maximum dose of 0.4 mg/day.
Most Common Adverse Reactions (incidence of ≥ 5% and at least twice the rate of placebo): somnolence, fatigue, decreased appetite, dizziness.
Adverse Reactions Leading to Discontinuation: There was one patient in the clonidine hydrochloride extended-release + stimulant (group (1%) who discontinued because of an adverse event (severe bradyphrenia, with severe fatigue).
Commonly observed adverse reactions (incidence of ≥2% in the treatment group and greater than the rate on placebo) during the treatment period are listed in Table 3.
Table 3: Common Adverse Reactions Occurring in ≥2% of Patients Treated with Clonidine Hydrochloride Extended-Release Tablets and Greater than the Rate of Placebo in the Flexible-Dose Adjunctive to Stimulant Therapy Trial -Treatment Period (Study 2)
Preferred Term
Clonidine hydrochloride extended-release tablets + Stimulant
N=102
(%)
PBO+Stimulant
N=96
(%)
PSYCHIATRIC DISORDERS
Somnolence+
Aggression
Affect Lability
Emotional Disorder
19
2
2
2
7
1
1
0
GENERAL DISORDERS
Fatigue†
Irritability
14
2
4
7
NERVOUS SYSTEM DISORDERS
Headache
Insomnia
7
4
12
3
GASTRO-INTESTINAL DISORDERS
Upper Abdominal Pain
7
4
RESPIRATORY DISORDERS
Nasal Congestion
2
2
METABOLISM AND NUTRITION DISORDERS
Decreased Appetite
6
3
CARDIAC DISORDERS
Dizziness
5
1
+Somnolence includes the terms: "somnolence" and "sedation"
† Fatigue includes the terms "fatigue" and "lethargy"
Commonly observed adverse reactions (incidence of ≥2% in the treatment group and greater than the rate on placebo) during the taper period are listed in Table 4.
Table 4: Common Adverse Reactions Occurring in ≥2% of Patients Treated with Clonidine Hydrochloride Extended-Release Tablets and Greater than the Rate of Placebo in the Flexible-Dose Adjunctive to Stimulant Therapy Trial -Taper Period+ (Study 2)
Preferred Term
Clonidine hydrochloride extended-release tablets + Stimulant
N=102
(%)
Placebo+Stimulant
N=96
(%)
Nasal Congestion
4
2
Headache
3
1
Irritability
3
2
Throat Pain
3
1
Gastroenteritis Viral
2
0
Rash
2
0
+Taper Period: weeks 6-8
Adverse Reactions Leading to Discontinuation
Thirteen percent (13%) of patients receiving clonidine hydrochloride extended-release discontinued from the pediatric monotherapy study due to adverse reactions, compared to 1% in the placebo group. The most common adverse reactions leading to discontinuation of clonidine hydrochloride extended-release monotherapy treated patients were somnolence/sedation (5%) and fatigue (4%).
Effect on Blood Pressure and Heart Rate
In patients that completed 5 weeks of treatment in a controlled, fixed-dose monotherapy study in pediatric patients, during the treatment period the maximum placebo-subtracted mean change in systolic blood pressure was -4.0 mmHg on clonidine hydrochloride extended-release 0.2 mg/day and -8.8 mmHg on clonidine hydrochloride extended-release 0.4 mg/day. The maximum placebo-subtracted mean change in diastolic blood pressure was -4.0 mmHg on clonidine hydrochloride extended-release 0.2 mg/day and -7.3 mmHg on clonidine hydrochloride extended-release 0.4 mg/day. The maximum placebo-subtracted mean change in heart rate was ‑4.0 beats per minute on clonidine hydrochloride extended-release 0.2 mg/day and -7.7 beats per minute on clonidine hydrochloride extended-release 0.4 mg/day.
During the taper period of the fixed-dose monotherapy study the maximum placebo-subtracted mean change in systolic blood pressure was +3.4 mmHg on clonidine hydrochloride extended‑release 0.2 mg/day and -5.6 mmHg on clonidine hydrochloride extended-release 0.4 mg/day. The maximum placebo-subtracted mean change in diastolic blood pressure was +3.3 mmHg on clonidine hydrochloride extended-release 0.2 mg/day and -5.4 mmHg on clonidine hydrochloride extended-release 0.4 mg/day. The maximum placebo-subtracted mean change in heart rate was -0.6 beats per minute on clonidine hydrochloride extended-release 0.2 mg/day and -3.0 beats per minute on clonidine hydrochloride extended-release 0.4 mg/day.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of clonidine hydrochloride extended-release tablets (and excludes those already mentioned in Section 6.1). Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Psychiatric: hallucinations
Cardiovascular: Q-T prolongation
-
7 DRUG INTERACTIONS
The interactions of ONYDA XR with co-administration of other drugs have not been studied. The drug interaction data provided in this section is based on oral immediate-release clonidine formulations.
Table 5 displays clinically important drug interactions with ONYDA XR.
Table 5: Clinically Important Drug Interactions with ONYDA XR
Antihypertensive drugs
Clinical Implication
Concomitant use of antihypertensive drugs with clonidine potentiates the hypotensive effects of clonidine.
Intervention
Monitor blood pressure and heart rate, and adjust dosage of ONYDA XR accordingly in patients treated concomitantly with antihypertensives [see Warnings and Precautions (5.1)].
CNS depressants
Clinical Implication
Concomitant use of CNS depressants with clonidine potentiates the sedating effects [see Warnings and Precautions (5.2)].
Intervention
Avoid concomitant use of CNS depressants with ONYDA XR.
Drugs that affect sinus node function or AV node conduction (e.g., digitalis, calcium channel blockers, beta blockers)
Clinical Implication
Concomitant use of drugs that affect sinus node function or AV node conduction with clonidine potentiate bradycardia and risk of AV block [see Warnings and Precautions (5.5)].
Intervention
Avoid concomitant use of drugs that affect sinus node function or AV node conduction with ONYDA XR.
Tricyclic antidepressants
Clinical Implication
Concomitant use of tricyclic antidepressants with clonidine can increase blood pressure and may counteract the hypotensive effects of clonidine.
Intervention
Monitor blood pressure and adjust dosage of ONYDA XR as needed.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to ADHD medications, including ONYDA XR, during pregnancy. Healthcare providers are encouraged to advise patients to register by calling the National Pregnancy Registry for Psychiatric Medications at 1-866-961-2388 or visiting online at https://womensmentalhealth.org/adhd-medications/.
Risk Summary
Prolonged experience with clonidine in pregnant women over several decades, based on published literature, including controlled trials, a retrospective cohort study and case reports, have not identified a drug associated risk of major birth defects, miscarriage, and adverse maternal or fetal outcomes. In animal embryofetal studies, increased resorptions were seen in rats and mice administered oral clonidine hydrochloride from implantation through organogenesis at 10 and 5 times, respectively, the maximum recommended human dose (MRHD) given to adolescents on a mg/m2 basis. No developmental effects were seen in rabbits administered oral clonidine hydrochloride during organogenesis at doses up to 3 times the MRHD (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriages in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Oral administration of clonidine hydrochloride to pregnant rabbits during the period of embryo/fetal organogenesis at doses of up to 80 mcg/kg/day (approximately 3 times the oral maximum recommended daily dose [MRHD] of 0.4 mg/day given to adolescents on a mg/m2 basis) produced no developmental effects. In pregnant rats, however, doses as low as 15 mcg/kg/day (1/3 the MRHD given to adolescents on a mg/m2 basis) were associated with increased resorptions in a study in which dams were treated continuously from 2 months prior to mating and throughout gestation. Increased resorptions were not associated with treatment at the same or at higher dose levels (up to 3 times the MRHD) when treatment of the dams was restricted to gestation days 6-15. Increases in resorptions were observed in both rats and mice at 500 mcg/kg/day (10 and 5 times the MRHD in rats and mice, respectively) or higher when the animals were treated on gestation days 1-14; 500 mcg/kg/day was the lowest dose employed in this study.
8.2 Lactation
Risk Summary
Based on published lactation studies, clonidine hydrochloride is present in human milk at relative infant doses ranging from 4.1% to 8.4% of the maternal weight-adjusted dosage. Although in most cases, there were no reported adverse effects in breastfed infants exposed to clonidine, there is one case report of sedation, hypotonia, and apnea in an infant exposed to clonidine through breast milk. If an infant is exposed to clonidine through breastmilk, monitor for symptoms of hypotension and bradycardia, such as sedation, lethargy, tachypnea and poor feeding (see Clinical Considerations). The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ONYDA XR and any potential adverse effects on the breastfed child from ONYDA XR or from the underlying maternal condition. Exercise caution when ONYDA XR is administered to a nursing woman.
Clinical Considerations
Monitor breastfeeding infants exposed to ONYDA XR through breast milk for symptoms of hypotension and/or bradycardia such as sedation, lethargy, tachypnea, and poor feeding.
8.3 Females and Males of Reproductive Potential
Infertility
Findings in animal studies revealed that ONYDA XR may impair fertility in females and males of reproductive potential [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and efficacy of clonidine hydrochloride extended-release in the treatment of ADHD have been established in pediatric patients 6 to 17 years of age. Use of clonidine hydrochloride extended-release in pediatric patients 6 to 17 years of age is supported by three adequate and well-controlled studies; a short-term, placebo-controlled monotherapy trial, a short-term adjunctive therapy trial and a longer-term randomized monotherapy trial [see Clinical Studies (14)]. Safety and efficacy in pediatric patients below the age of 6 years has not been established.
Juvenile Animal Data
In studies in juvenile rats, clonidine hydrochloride alone or in combination with methylphenidate had an effect on bone growth at clinically relevant doses and produced a slight delay in sexual maturation in males at 3 times the maximum recommended human dose (MRHD) for clonidine and methylphenidate.
In a study where juvenile rats were treated orally with clonidine hydrochloride from day 21 of age to adulthood, a slight delay in onset of preputial separation (delayed sexual maturation) was seen in males treated with 300 mcg/kg/day, which is approximately 3 times the MRHD of 0.4 mg/day on a mg/m2 basis. The no-effect dose was 100 mcg/kg/day, which is approximately equal to the MRHD. There was no drug effects on fertility or on other measures of sexual or neurobehavioral development.
In a study where juvenile rats were treated with clonidine alone (300 mcg/kg/day) or in combination with methylphenidate (10 mg/kg/day in females and 50/30 mg/kg/day in males; the dose was lowered from 50 to 30 mg/kg/day in males due to self-injurious behavior during the first week of treatment) from day 21 of age to adulthood, decreases in bone mineral density and mineral content were observed in males treated with 300 mcg/kg/day clonidine alone and in combination with 50/30 mg/kg/day methylphenidate and a decrease in femur length was observed in males treated with the combination at the end of the treatment period. These doses are approximately 3 times the MRHD of 0.4 mg/day clonidine and 54 mg/day methylphenidate on a mg/m2 basis. All these effects in male were not reversed at the end of a 4-week recovery period. In addition, similar findings were seen in males treated with a lower dose of clonidine (30 mcg/kg/day) in combination with 50 mg/kg/day of methylphenidate and a decrease in femur length was observed in females treated with clonidine alone at the end of the recovery period. These effects were accompanied by a decrease in body weight gain in treated animals during the treatment period but the effect was reversed at the end of the recovery period. A delay in preputial separation (sexual maturation) was observed in males treated with the combination treatment of 300 mcg/kg/day clonidine and 50/30 mg/kg/day methylphenidate. There was no effect on reproduction or sperm analysis in these males.
8.6 Renal Impairment
The impact of renal impairment on the pharmacokinetics of clonidine in pediatric patients has not been assessed. The initial dosage of ONYDA XR should be based on degree of impairment. Monitor patients carefully for hypotension and bradycardia, and titrate to higher doses cautiously. Since only a minimal amount of clonidine is removed during routine hemodialysis, there is no need to give supplemental ONYDA XR following dialysis.
-
10 OVERDOSAGE
Hypertension may develop early and may be followed by hypotension, bradycardia, respiratory depression, hypothermia, drowsiness, decreased or absent reflexes, weakness, irritability and miosis. The frequency of CNS depression may be higher in pediatric patients than adults. Large overdoses may result in reversible cardiac conduction defects or dysrhythmias, apnea, coma and seizures. Signs and symptoms of overdose generally occur within 30 minutes to two hours after exposure.
Consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdose management recommendations.
-
11 DESCRIPTION
ONYDA XR contains clonidine hydrochloride, a centrally acting alpha2-adrenergic agonist. Clonidine hydrochloride is an imidazoline derivative and exists as a mesomeric compound. The chemical name is 2-[(2,6-dichlorophenyl)imino]imidazolidine hydrochloride. The following is the structural formula:
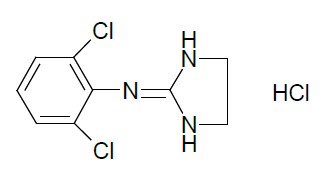
The molecular formula of clonidine hydrochloride is C9H9Cl2N3HCl and the molecular weight is 266.5. The pKa is 8.05.
Clonidine hydrochloride is an odorless, bitter, white to almost white, crystalline powder soluble in water and alcohol. The pH of a 5% solution in water is between 3.5 and 5.5.
ONYDA XR is an extended-release suspension for oral administration. Each mL of ONYDA XR contains 0.09 mg clonidine equivalent to 0.1 mg clonidine hydrochloride (0.095 mg clonidine hydrochloride complexed with sodium polystyrene sulfonate and 0.005 mg clonidine hydrochloride). The pH of ONYDA XR is between 2.8 and 4.
The inactive ingredients are anhydrous citric acid, edetate disodium, glycerin, modified starch, methylparaben, orange flavor, polyvinyl acetate dispersion 30%, povidone, polysorbate 80, propylparaben, purified water, sucrose, sodium polystyrene sulfonate, triacetin, xanthan gum.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Clonidine stimulates alpha2-adrenergic receptors in the brain. Clonidine is not a central nervous system stimulant. The mechanism of action of clonidine in ADHD is not known.
12.2 Pharmacodynamics
Clonidine is a known antihypertensive agent. By stimulating alpha2-adrenergic receptors in the brain stem, clonidine reduces sympathetic outflow from the central nervous system and decreases peripheral resistance, renal vascular resistance, heart rate, and blood pressure.
12.3 Pharmacokinetics
Immediate-release clonidine hydrochloride, extended-release clonidine hydrochloride tablets, and ONYDA XR have different pharmacokinetic characteristics. Dose substitution on a milligram for milligram basis will result in differences in exposures [see Dosage and Administration (2.3)].
Absorption
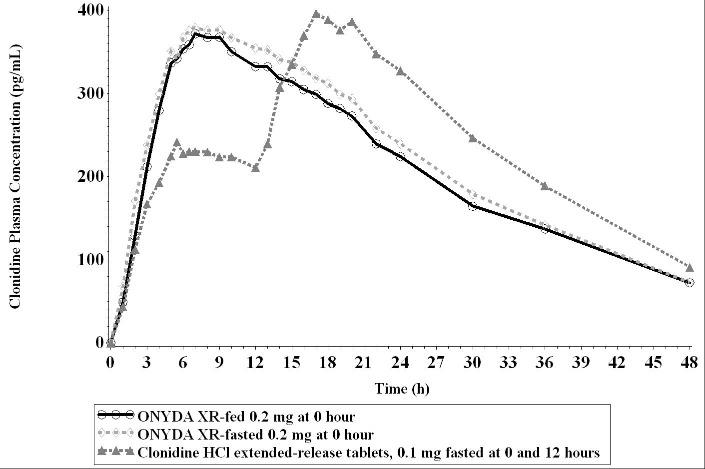
Following a single 0.2 mg dose of ONYDA XR in 20 healthy adult subjects under fasting conditions in a crossover study, the median (range) time to peak plasma concentrations (Tmax) for clonidine was 7.50 (4 –17) hours after dosing. Peak concentration (Cmax) was 95.6% of the Cmax of clonidine extended-release tablet 0.1 mg administered at 0 and 12 hours under fasting conditions. The relative bioavailability of ONYDA XR compared with an equal dose of clonidine extended-release tablet was 96.1%.
After oral administration of 0.2 mg of ONYDA XR once daily over 5 days under fasted conditions in healthy adult subjects, the peak steady state plasma concentration (Cmax.ss) was 107.9%, and steady state relative bioavailability (AUCt, ss) was 97.7% compared with 0.1 mg of clonidine extended-release tablet administered twice daily under fasting conditions. The minimum concentration at steady state (Cmin,ss) of ONYDA XR was about 26% lower than that of the equal dose of clonidine extended-release tablet.
Following oral administration of an immediate release formulation in healthy adult subjects, plasma clonidine concentration peaks in approximately 3 to 5 hours.
A comparison across studies suggests that the Cmax is 50% lower for clonidine hydrochloride extended-release tablets compared to immediate-release clonidine hydrochloride.
Effect of Food
Food had no effect on plasma exposures of clonidine after administration of ONYDA XR (see Figure 1).
Figure 1: Mean Clonidine Concentration-Time Profiles After Single Dose Administration
Elimination
The plasma half-life of immediate-release clonidine ranges from 12 to 16 hours. The half-life increases up to 41 hours in patients with severe impairment of renal function.
Metabolism
About 50% of the absorbed dose is metabolized in the liver.
Excretion
Following oral administration about 40% to 60% of the absorbed dose is recovered in the urine as unchanged drug in 24 hours. Although studies of the effect of renal impairment and studies of clonidine excretion have not been performed with ONYDA XR, results are expected to be similar to those of the immediate-release formulation.
Specific Populations
Pediatric patients
Plasma clonidine concentrations in pediatric patients 6 to 17 years (0.1 mg twice daily and 0.2 mg twice daily of clonidine hydrochloride extended-release tablets) with ADHD are greater than those of adults with hypertension, with pediatric patients 6 to 17 years receiving higher doses on a mg/kg basis. Body weight normalized clearance (CL/F) in pediatric patients aged 6 to 17 years was higher than CL/F observed in adults with hypertension. Clonidine concentrations in plasma increased with increases in dose over the dose range of 0.2 to 0.4 mg/day. Clonidine CL/F was independent of dose administered over the 0.2 to 0.4 mg/day dose range. Clonidine CL/F appeared to decrease slightly with increases in age over the range of 6 to 17 years, and females had a 23% lower CL/F than males. The incidence of "sedation-like" events (somnolence and fatigue) appeared to be independent of clonidine dose or concentration within the studied dose range in the titration study. Results from the add-on study showed that clonidine CL/F was 11% higher in patients who were receiving methylphenidate and 44% lower in those receiving amphetamine compared to subjects not on adjunctive therapy.
Drug Interaction Studies
Alcohol: In an in vitro alcohol-induced dose dumping study, a significantly faster and more variable ONYDA XR drug release was observed in the presence of 20% alcohol, but not with 5% or 10% alcohol, when compared to 0% alcohol.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis and Impairment of Fertility
Carcinogenesis
Clonidine hydrochloride was not carcinogenic when administered in the diet of rats (for up to 132 weeks) or mice (for up to 78 weeks) at doses of up to 1,620 (male rats), 2,040 (female rats), or 2,500 (mice) mcg/kg/day. These doses are approximately 20, 25, and 15 times, respectively, the maximum recommended human dose (MRHD) of 0.4 mg/day on a mg/m2 basis.
Mutagenesis
There was no evidence of genotoxicity in the Ames test for mutagenicity or mouse micronucleus test for clastogenicity.
Impairment of Fertility
In a reproduction study fertility of female rats appeared to be adversely affected at dose levels of 500 and 2,000 mcg/kg/day (10 and 40 times the MRHD on a mg/m2 basis). Lower doses have not been adequately evaluated and a no adverse effect level could not be established.
-
14 CLINICAL STUDIES
The efficacy of ONYDA XR for the treatment of ADHD in pediatric patients 6 years of age and older is based upon adequate and well-controlled studies of clonidine hydrochloride extended-release tablets (referred to as “clonidine hydrochloride extended-release” in this section). The efficacy results of these adequate and well-controlled studies of clonidine hydrochloride extended-release tablets are presented below.
Efficacy of clonidine hydrochloride extended-release in the treatment of ADHD was established in pediatric patients 6 to 17 years in:
- One short-term, placebo-controlled monotherapy trial (Study 1)
- One short-term adjunctive therapy to psychostimulants trial (Study 2)
- One randomized withdrawal trial as monotherapy (Study 3)
Short-term Monotherapy and Adjunctive Therapy to Psychostimulant Studies for ADHD
The efficacy of clonidine hydrochloride extended-release in the treatment of ADHD was established in 2 (one monotherapy and one adjunctive therapy) placebo-controlled trials in pediatric patients aged 6 to 17 years, who met DSM-IV criteria of ADHD hyperactive or combined hyperactive/inattentive subtypes. Signs and symptoms of ADHD were evaluated using the investigator administered and scored ADHD Rating Scale-IV-Parent Version (ADHDRS-IV) total score including hyperactive/impulsivity and inattentive subscales. Study 1 was an 8-week randomized, double-blind, placebo-controlled, fixed dose study of pediatric patients 6 to 17 years (N=236) with a 5-week primary efficacy endpoint. Patients were randomly assigned to one of the following three treatment groups: clonidine hydrochloride extended-release 0.2 mg/day (N=78), clonidine hydrochloride extended-release 0.4 mg/day (N=80), or placebo (N=78). Dosing for the clonidine hydrochloride extended-release groups started at 0.1 mg/day and was titrated in increments of 0.1 mg/week to their respective dose (as divided doses). Patients were maintained at their dose for a minimum of 2 weeks before being gradually tapered down to 0.1 mg/day at the last week of treatment. At both doses, improvements in ADHD symptoms were statistically significantly superior in clonidine hydrochloride extended-release-treated patients compared with placebo-treated patients at the end of 5 weeks as measured by the ADHDRS-IV total score (Table 6). Study 2 was an 8-week randomized, double-blind, placebo-controlled, flexible dose study in pediatric patients 6 to 17 years (N=198) with a 5-week primary efficacy end point. Patients had been treated with a psychostimulant (methylphenidate or amphetamine) for four weeks with inadequate response. Patients were randomly assigned to one of two treatment groups: clonidine hydrochloride extended-release adjunct to a psychostimulant (N=102) or psychostimulant alone (N=96). The clonidine hydrochloride extended-release dose was initiated at 0.1 mg/day and doses were titrated in increments of 0.1 mg/week up to 0.4 mg/day, as divided doses, over a 3-week period based on tolerability and clinical response. The dose was maintained for a minimum of 2 weeks before being gradually tapered to 0.1 mg/day at the last week of treatment. ADHD symptoms were statistically significantly improved in clonidine hydrochloride extended-release plus stimulant group compared with the stimulant alone group at the end of 5 weeks as measured by the ADHDRS-IV total score (Table 6).
Table 6: Short-Term Trials
Study Number
Treatment Group
Primary Efficacy Measure: ADHDRS-IV Total Score
Mean Baseline Score (SD)
LS Mean Change from Baseline (SE)
Placebo-subtracted Differencea (95% CI)
Study 1
Clonidine hydrochloride extended-release tablets
(0.2 mg/day)
43.8 (7.47)
-15.0 (1.38)
-8.5 (-12.2, -4.8)
Clonidine hydrochloride extended-release tablets (0.4 mg/day)
44.6 (7.73)
-15.6 (1.33)
-9.1 (-12.8, -5.5)
Placebo
45.0 (8.53)
-6.5 (1.35)
----------
Study 2
Clonidine hydrochloride extended-release tablets (0.4 mg/day) + Psychostimulant
38.9 (6.95)
-15.8 (1.18)
-4.5 (-7.8, -1.1)
Psychostimulant alone
39.0 (7.68)
-11.3 (1.24)
----------
a Difference (drug minus placebo) in least-squares mean change from baseline. SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: unadjusted confidence interval.
Maintenance Monotherapy for ADHD
Study 3 was a double-blind, placebo-controlled, randomized-withdrawal study in pediatric patients 6 to 17 years (n=253) with DSM-IV-TR diagnosis of ADHD. The study consisted of a 10-week, open-label phase (4 weeks of dose optimization and 6 weeks of dose maintenance), a 26-week double-blind phase, and a 4-week taper-down and follow-up phase. All patients were initiated at 0.1 mg/day and increased at weekly intervals in increments of 0.1 mg/day until reaching personalized optimal dose (0.1, 0.2, 0.3 or 0.4 mg/day, as divided doses). Eligible patients had to demonstrate treatment response as defined by ≥ 30% reduction in ADHD-RS-IV total score and a Clinical Global Impression-Improvement score of 1 or 2 during the open label phase. Patients who sustained treatment response (n=135) until the end of the open label phase were randomly assigned to one of the two treatment groups, clonidine hydrochloride extended-release (N=68) and Placebo (N=67), to evaluate the long-term efficacy of maintenance dose of clonidine hydrochloride extended-release in the double-blind phase. The primary efficacy endpoint was the percentage of patients with treatment failure defined as a ≥ 30% increase (worsening) in ADHD-RS-IV total score and ≥ 2 points increase (worsening) in Clinical Global Impression – Severity Scale in 2 consecutive visits or early termination for any reason. A total of 73 patients experienced treatment failure in the double-blind phase: 31 patients (45.6%) in the clonidine hydrochloride extended-release group and 42 patients (62.7%) in the placebo group, with a statistically significant difference in the primary endpoint favoring clonidine (Table 7). The cumulative proportion of patients with treatment failure over time during the double-blind phase is displayed in Figure 2.
Table 7: Treatment Failure: Double-Blind Full Analysis Set (Study 3)
Study 3
Double-Blind Full Analysis Set
Clonidine Hydrochloride Extended-Release Tablets
Placebo
Number of patients
68
67
Number of treatment failures
31 (45.6%)
42 (62.7%)
Basis of Treatment Failure
Clinical criteriaa,b
11 (16.2%)
9 (13.4%)
Lack of efficacyc
1 (1.5%)
3 (4.5%)
Withdrawal of informed assent/consent
4 (5.9%)
20 (29.9%)
Other early terminations
15 (22.1%)
10 (14.9%)
ADHD-RS-IV = Attention Deficit Hyperactivity Disorder-Rating Scale-4th edition; CGI-S = Clinical Global Impression-Severity
a At the same 2 consecutive visits a (1) 30% or greater reduction in ADHD-RS-IV, and (2) 2-point or more increase in CGI-S.
bTwo patients (1 placebo and 1 clonidine hydrochloride extended-release tablets) withdrew consent, but met the clinical criteria for treatment failure.
cThree patients (all placebo) discontinued the study due to treatment failure, but met only the criterion for ADHD-RS-IV.
Figure 2: Kaplan-Meier Estimation of Cumulative Proportion of Pediatric Patients (6 to 17 Years) with Treatment Failure (Study 3)
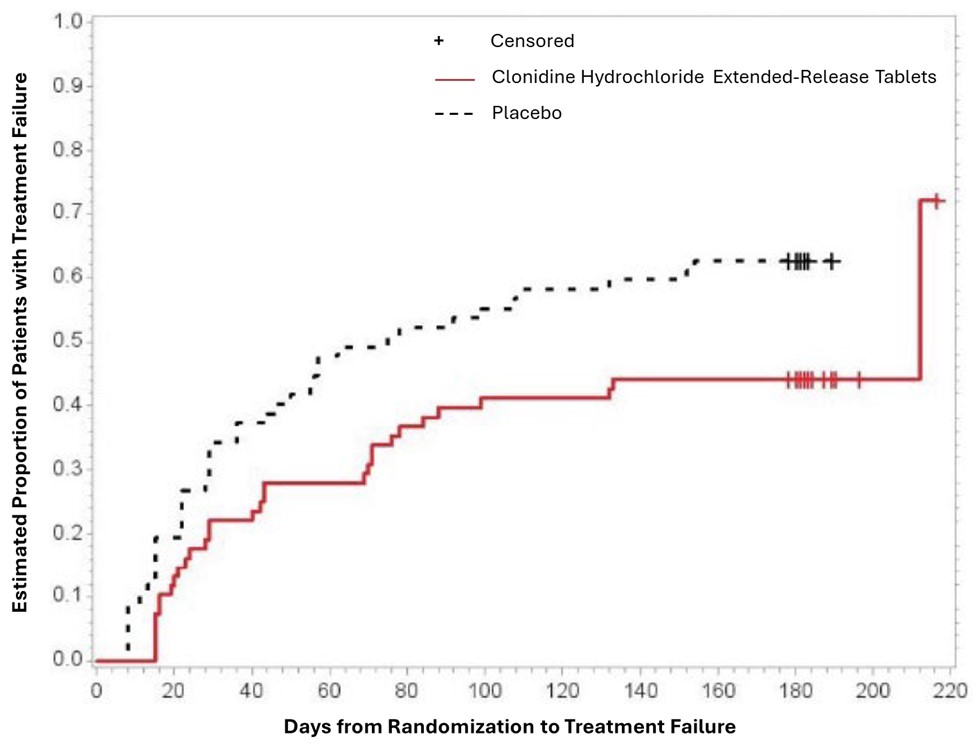
Figure 2
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
ONYDA XR (clonidine hydrochloride) extended-release oral suspension 0.1 mg/mL is a light beige to tan viscous suspension.
ONYDA XR is supplied in a carton. Each carton contains one bottle with a child resistant closure, an oral dosing dispenser(s), and a press in bottle adapter(s).
Bottle of 30 mL NDC: 24478-148-03 One (1) oral dosing dispenser and one (1) press in bottle adapter Bottle of 60 mL NDC: 24478-148-04 One (1) oral dosing dispenser and one (1) press in bottle adapter Bottle of 120 mL NDC: 24478-148-02 Two (2) oral dosing dispensers and two (2) press in bottle adapters Storage and Handling
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Protect from light.
Store and dispense ONYDA XR in the original bottle. Dispense with bottle adapter and oral dosing dispenser supplied in the carton.
For the bottles of 30 mL and 60 mL, discard any unused ONYDA XR 30 days after first opening the bottle.
For the 120 mL bottle, discard any unused ONYDA XR 60 days after first opening the bottle.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Dosage and Administration
Advise patients that ONYDA XR may be taken with or without food. When initiating treatment, provide titration instructions [see Dosage and Administration (2.1)].
Administration Instructions
Instruct patients to read the “Instructions for Use” for complete administration instructions [see Dosage and Administration (2.2)].
Advise patients to:
- firmly insert the bottle adapter into the bottle and do not remove the bottle adapter once inserted. Use the oral dispenser provided with ONYDA XR.
- gently shake ONYDA XR with a smooth up and down motion (to avoid foaming) for at least 10 seconds before each administration.
- For the bottles of 30 mL and 60 mL, discard any unused ONYDA XR 30 days after first opening the bottle.
- For the 120 mL bottle, discard any unused ONYDA XR 60 days after first opening the bottle.
Missed Dose
If patients miss a dose of ONYDA XR, advise them to skip the dose and take the next dose as scheduled and not to take more than the prescribed total daily amount of ONYDA XR in any 24‑hour period [see Dosage and Administration (2.5)].
Hypotension/Bradycardia
Advise patients who have a history of syncope or may have a condition that predisposes them to syncope, such as hypotension, orthostatic hypotension, bradycardia, or dehydration, to avoid becoming dehydrated or overheated [see Warnings and Precautions (5.1)].
Sedation and Somnolence
Instruct patients to use caution when driving a car or operating heavy equipment until they know how they will respond to treatment with ONYDA XR. Also advise patients to avoid the use of ONYDA XR with other centrally active depressants and with alcohol [see Warnings and Precautions (5.2)].
Rebound Hypertension
Advise patients not to discontinue ONYDA XR abruptly. Inform patients and caregivers that pediatric patients with gastrointestinal illnesses that lead to vomiting may be at increased risk for rebound hypertension [see Warnings and Precautions (5.3)].
Allergic Reactions
Advise patients to discontinue ONYDA XR and seek immediate medical attention if any signs or symptoms of a hypersensitivity reaction occur, such as generalized rash, urticaria, or angioedema [see Warnings and Precautions (5.4)].
Pregnancy Registry
Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in patients exposed to ONYDA XR during pregnancy [see Use in Specific Populations (8.1)].
Lactation
Advise breastfeeding women using ONYDA XR to monitor infants for excess sedation, decreased muscle tone, and respiratory depression and to seek medical care if they notice these signs [see Use in Specific Populations (8.2)].
Fertility
Advise females and males of reproductive potential that ONYDA XR may impair fertility [see Use in Specific Populations (8.3) and Nonclinical Toxicology (13.1)].
Manufactured by/Distributed by:
Tris Pharma, Inc.
Monmouth Junction, NJ 08852
LB8735
Rev. 05
-
PATIENT INFORMATION
ONYDATM XR (oh-nee-dah)
(clonidine hydrochloride)
extended-release oral suspension
What is ONYDA XR?
ONYDA XR is a prescription medicine used for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) alone or with certain other ADHD medicines in children 6 years of age and older.
It is not known if ONYDA XR is safe and effective in children under 6 years of age.
Who should not take ONYDA XR?
Do not take ONYDA XR if you are allergic to clonidine. See the end of this Patient Information for a complete list of ingredients in ONYDA XR.
Before taking ONYDA XR, tell your healthcare provider about all of your medical conditions, including if you:
- have kidney problems
- have low or high blood pressure
- have a history of passing out (syncope)
- have heart problems, including slow heart rate or other heart rhythm problems
- had a stroke or have stroke symptoms
- had an allergic reaction after taking clonidine through your skin in a transdermal system (patch)
- are pregnant or plan to become pregnant. It is not known if ONYDA XR will harm the unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant.
- There is a pregnancy registry for females who are exposed to ADHD medications, including ONYDA XR, during pregnancy. The purpose of the registry is to collect information about the health of females exposed to ONYDA XR and their baby. If you become pregnant during treatment with ONYDA XR, talk to your healthcare provider about registering with the National Pregnancy Registry of ADHD Medications at 1-866-961-2388 or visit online at https://womensmentalhealth.org/adhdmedications/
- are breastfeeding or plan to breastfeed. ONYDA XR passes into the breast milk. Babies who are breastfed during treatment with ONYDA XR may become very sleepy, develop relaxed or floppy muscles, and develop trouble breathing. Call the baby’s healthcare provider if the baby is breastfed during treatment with ONYDA XR and develops any of these symptoms. Talk to your healthcare provider about the best way to feed your baby if you take ONYDA XR.
Tell your healthcare provider about all of the medicines that you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
ONYDA XR and certain other medicines may affect each other causing serious side effects. Especially tell your healthcare provider if you take:
anti-depression medicines other medicines for ADHD
heart or blood pressure medicines medicines that cause sleepiness (sedation)
other medicines that contain clonidineAsk your healthcare provider or pharmacist if you are not sure if your medicine is listed above.
Know the medicines that you take. Keep a list of your medicines with you to show your healthcare provider and pharmacist when you get a new medicine.
How should I take ONYDA XR?
- Take ONYDA XR 1 time daily at bedtime with or without food.
- Take ONYDA XR exactly as your healthcare provider tells you.
- Your healthcare provider may change your dose of ONYDA XR. Do not change your dose of ONYDA XR without talking to your healthcare provider.
- If you are taking another clonidine medicine, stop taking it before you start treatment with ONYDA XR.
- The dose of ONYDA XR is not the same as other clonidine medicines. Do not change between ONYDA XR and other clonidine medicines unless your healthcare provider tells you.
- Do not stop taking ONYDA XR without talking to your healthcare provider.
- If you miss a dose of ONYDA XR, skip the missed dose and take the next dose at their regular scheduled time. Do not take more ONYDA XR in a 24-hour period than your healthcare provider prescribed for your daily dose.
- For the bottles of 30 mL and 60 mL, throw away (discard of) any remaining ONYDA XR if you have not used it 30 days after first opening the bottle.
- For the 120 mL bottle, throw away (discard of) any remaining ONYDA XR if you have not used it 60 days after first opening the bottle.
- See the detailed Instructions for Use for information on how to take a dose of ONYDA XR.
If you take too much ONYDA XR, call your healthcare provider or Poison Help line at 1-800-222-1222, or go to the nearest hospital emergency room right away.
What should I avoid while taking ONYDA XR?
- Do not become dehydrated or too hot (overheated) to decrease your chance of passing out during treatment with ONYDA XR.
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how ONYDA XR affects you because ONYDA XR can cause sleepiness and tiredness that could cause slow reaction times.
- Do not drink alcohol or take other medicines that make you sleepy or dizzy during treatment with ONYDA XR until you talk with your healthcare provider. Taking ONYDA XR with alcohol or medicines that cause sleepiness or dizziness may make your sleepiness or dizziness worse.
- Do not suddenly stop ONYDA XR. Tell your healthcare provider if you have been vomiting and cannot take ONYDA XR, you may be at risk for rebound hypertension.
What are possible side effects of ONYDA XR?
ONYDA XR may cause serious side effects, including:
- Decreased blood pressure and heart rate. ONYDA XR can decrease your blood pressure and heart rate, which can increase your chance of passing out (syncope). If you have a history of passing out or have other medical problems or take other medicines that increase your risk of passing out, your risk is higher. Your healthcare provider should check your heart rate and blood pressure before starting treatment and regularly during treatment with ONYDA XR. See “What should I avoid while taking ONYDA XR?”
- Sleepiness and tiredness that could cause slow reaction times (sedation and somnolence). See “What should I avoid while taking ONYDA XR?”
- Rebound high blood pressure (hypertension). Suddenly stopping ONYDA XR can cause high blood pressure to return if you have a history of high blood pressure. Suddenly stopping ONYDA XR may also cause withdrawal symptoms including headache, increased heart rate, nausea, flushing or warm feeling, lightheadedness, tightness in your chest and nervousness or anxiety. Do not suddenly stop ONYDA XR treatment without first talking to your healthcare provider.
- Allergic reactions. You may develop an allergic reaction to ONYDA XR if you had an allergic reaction to clonidine taken through your skin in a patch. Stop taking ONYDA XR and call your healthcare provider right away or go to the nearest emergency room if you develop any signs or symptoms of an allergic reaction, including:
o skin rash o hives o swelling of the eyes, face, lips, or tongue
The most common side effects of ONYDA XR when used alone include:
falling asleep or sleepiness and tiredness that could cause slow reaction times nightmare constipation
irritability trouble sleeping dry mouthThe most common side effects of ONYDA XR when used with other ADHD medicines include:
falling asleep or sleepiness and tiredness that could cause slow reaction times decreased appetite dizziness
ONYDA XR may cause fertility problems in females and males, which may affect your ability to have a child. Talk to your healthcare provider if this is a concern for you.
These are not all of the possible side effects of ONYDA XR.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store ONYDA XR?
- Store ONYDA XR at room temperature between 68°F to 77°F (20°C to 25°C).
- Protect from light and keep ONYDA XR in the original container. Tightly close the child-resistant cap.
- For the bottles of 30 mL and 60 mL, throw away (discard of) any remaining ONYDA XR if you have not used it 30 days after first opening the bottle.
- For the 120 mL bottle, throw away (discard of) any remaining ONYDA XR if you have not used it 60 days after first opening the bottle.
Keep ONYDA XR and all medicines out of the reach of children.
General information about the safe and effective use of ONYDA XR.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ONYDA XR for a condition for which it was not prescribed. Do not give ONYDA XR to other people, even if they have the same symptoms that you have. It may harm them. You can also ask your pharmacist or healthcare provider for information about ONYDA XR that is written for health professionals.
What are the ingredients in ONYDA XR?
Active Ingredient: clonidine hydrochloride
Inactive Ingredients: anhydrous citric acid, edetate disodium, glycerin, modified starch, methylparaben, orange flavor, polyvinyl acetate dispersion 30%, povidone, polysorbate 80, propylparaben, purified water, sucrose, sodium polystyrene sulfonate, triacetin, and xanthan gum
Manufactured by/Distributed by:
Tris Pharma, Inc.
Monmouth Junction, NJ 08852
For more information about ONYDA XR call 1-732-940-0358.
Rev. 04
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised:04/2025
-
INSTRUCTIONS FOR USE
ONYDATM XR (oh-nee-dah)
(clonidine hydrochloride)
extended-release oral suspension
This Instructions for Use contains information on how to take ONYDA XR.
Important Information You Need to Know Before Taking ONYDA XR:
- ONYDA XR is for oral use only (taken by mouth).
- Take ONYDA XR with or without food.
- Use only the oral dosing syringe and bottle adapter that come with ONYDA XR to measure and take a dose of ONYDA XR.
- Shake the ONYDA XR bottle gently.
- Check the expiration date (EXP) on the carton label. Do not take ONYDA XR after the expiration date has passed. Call your healthcare provider or pharmacist if your medicine is expired.
- For the bottles of 30 mL and 60 mL, throw away (discard of) any remaining ONYDA XR if you have not used it 30 days after first opening the bottle.
- For the 120 mL bottle, throw away (discard of) any remaining ONYDA XR if you have not used it 60 days after first opening the bottle.
Supplies included in the ONYDA XR carton:
1 bottle of ONYDA XR Dosing Syringe(s) Bottle Adapter(s) 30 mL bottle 1 1 60 mL bottle 1 1 120 mL bottle 2 2 Figure A
Figure A
Step 1:
Preparing to take ONYDA XR:
- Wash and dry your hands (see Figure B).
- Remove the ONYDA XR bottle, 1 oral dosing syringe, and 1 bottle adapter from the carton.
Tell your pharmacist right away if you are missing any supplies from the carton.
Figure B
Step 2:
- Place the ONYDA XR bottle on a flat surface like a table or countertop and remove the child resistant cap by pressing down and turning counterclockwise (see Figure C).
Insert the bottle adapter (first time use only).
- Hold the bottle firmly and insert the ridged end of the bottle adapter into the neck of the bottle.
- Firmly push the bottle adapter all the way down with your thumb until the top of the adapter is aligned and flat (flush) with the top of the bottle (See Figure D).
- Do not remove the bottle adapter once it has been inserted into the bottle.
Figure C
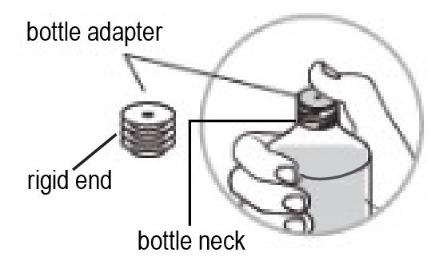
Figure D
Step 3:
- Put the cap back on the bottle and close it tightly by turning the cap clockwise (see Figure E).
- Shake the bottle gently with a smooth up and down motion to avoid foaming (see Figure F).
- Shake the bottle gently for at least 10 seconds before you take each dose.
Figure E
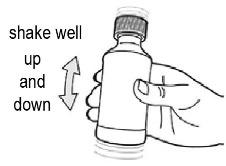
Figure F
Step 4:
Place the ONYDA XR bottle upright on the table or countertop. Open the cap again by pressing down and turn counterclockwise to remove the cap (see Figure G).
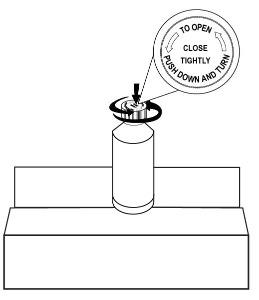
Figure G
Step 5:
- Check the ONYDA XR oral dosing syringe to find the right dose in milliliters (mL) that has been prescribed by the healthcare provider (see Figure H).
- Make sure the oral dosing syringe is dry.
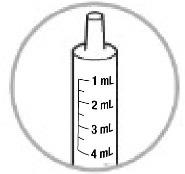
Figure H
Step 6:
- Insert the tip of the oral dosing syringe through the adapter into the bottle (see Figure I).
- Push the plunger all the way down (see Figure J).
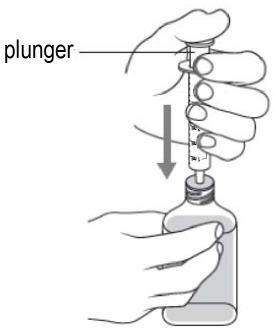
Figure I
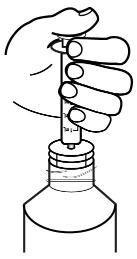
Figure J
Step 7:
Measuring the dose of ONYDA XR:
- With the tip of the oral dosing syringe in place in the adapter, hold the ONYDA XR bottle with 1 hand and turn the bottle upside down. Pull the plunger down until the white end of the plunger reaches the number of mL you need for the prescribed dose (see Figure K).
- Push and pull the plunger a few times to make sure that there are no air bubbles.
- Tap the barrel of the oral dosing syringe if needed to get rid of any air bubbles (see Figure L).
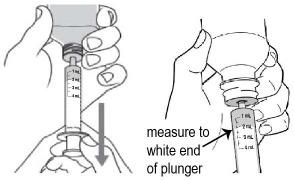
Figure K
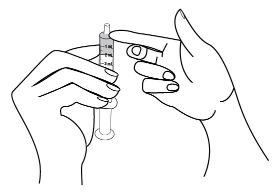
Figure L
Step 8:
- Turn the bottle over and place it upright on a table or countertop, then remove the oral dosing syringe from the bottle adapter (see Figure M).
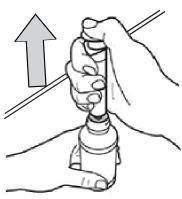
Figure M
Step 9:
- Check that the correct dose in mL was pulled up into the oral dosing syringe (see Figure N).
- If the dose is not correct:
- Insert the oral syringe tip back into the bottle and fully push in the plunger to the bottom of the syringe barrel so that all of the oral suspension flows back into the bottle.
- Repeat Steps 6 through 8.
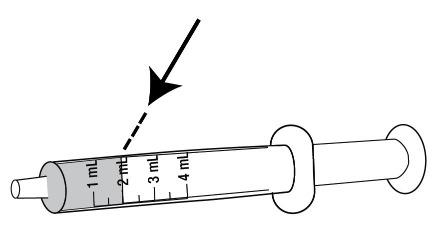
Figure N
Step 10:
Taking the dose of ONYDA XR:
- The child should be in a seated position before taking ONYDA XR.
- Tilt the head slightly upwards.
- Place the oral dosing syringe into the mouth and point it toward the cheek (see Figure O).
- Close the mouth tightly around the oral dosing syringe and slowly push the plunger all the way down to give the ONYDA XR dose (see Figure P).
- After all the medication has been taken, remove the oral dosing syringe from the mouth.
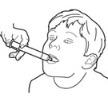
Figure O
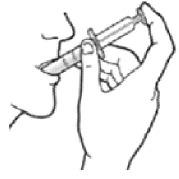
Figure P
Step 11:
Closing the bottle:
- Put the ONYDA XR cap back on the bottle and close the cap tightly by turning the cap clockwise (see Figure Q).
Figure Q
Step 12:
Cleaning up:
- Clean the oral dosing syringe after each use by rinsing with tap water (see Figure R).
- Allow the oral dosing syringe to dry and keep it in a safe place for the next use.
- Wash and dry your hands.
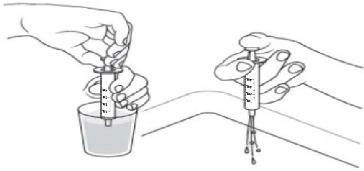
Figure R
Disposing of ONYDA XR:
- Throw away any unused or expired ONYDA XR in your household trash.
- For the bottles of 30 mL and 60 mL, throw away (discard of) any remaining ONYDA XR if you have not used it 30 days after first opening the bottle.
- For the 120 mL bottle, throw away (discard of) any remaining ONYDA XR if you have not used it 60 days after first opening the bottle.
Storing ONYDA XR:
- Store ONYDA XR at room temperature between 68°F to 77°F (20°C to 25°C).
- Protect from light and keep ONYDA XR in the original container. Tightly close the child-resistant cap.
Keep ONYDA XR and all medicines out of the reach of children.
Manufactured by/Distributed by:
Tris Pharma, Inc.
Monmouth Junction, NJ 08852
LB8730 Rev. 03
This Instructions for Use has been approved by the U.S. Food and Drug Administration
Revised: 02/2025
-
PRINCIPAL DISPLAY PANEL
NDC: 24478-148-01
ONYDATM XR(clonidine hydrochloride) extended-release oral suspension
0.1 mg
Shake Gently Before Use7 mL Rx only
-
PRINCIPAL DISPLAY PANEL
NDC: 24478-148-01
ONYDATM XR(clonidine hydrochloride) extended-release oral suspension
0.1 mg
Shake Gently Before Use
7 mL Rx only
-
PRINCIPAL DISPLAY PANEL
NDC: 24478-148-03
ONYDATM XR(clonidine hydrochloride) extended-release oral suspension
0.1 mg
Shake Gently Before Use
30 mL Rx only
-
PRINCIPAL DISPLAY PANEL
NDC: 24478-148-03
ONYDATM XR(clonidine hydrochloride) extended-release oral suspension
0.1 mg
Shake Gently Before Use
30 mL Rx only
-
INGREDIENTS AND APPEARANCE
ONYDA XR
clonidine hydrochloride suspension, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 24478-148 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLONIDINE HYDROCHLORIDE (UNII: W76I6XXF06) (CLONIDINE - UNII:MN3L5RMN02) CLONIDINE HYDROCHLORIDE 0.1 mg in 1 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) POLYVINYL ACETATE (UNII: 32K497ZK2U) POVIDONE (UNII: FZ989GH94E) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM POLYSTYRENE SULFONATE (UNII: 1699G8679Z) SUCROSE (UNII: C151H8M554) TRIACETIN (UNII: XHX3C3X673) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color brown (light beige to tan) Score Shape Size Flavor ORANGE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 24478-148-01 7 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/13/2024 2 NDC: 24478-148-02 120 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/13/2024 03/08/2025 3 NDC: 24478-148-03 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/13/2024 4 NDC: 24478-148-04 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/13/2024 03/08/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA217645 08/13/2024 Labeler - NextWave Pharmaceuticals, Inc (008816703)
Trademark Results [ONYDA XR]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ONYDA XR 98698414 not registered Live/Pending |
TRIS PHARMA, INC. 2024-08-14 |
 ONYDA XR 98573533 not registered Live/Pending |
TRIS PHARMA, INC. 2024-05-29 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.