PLUS153 TOOTHPAST E- sodium monofluorophosphate paste, dentifrice
PLUS153 Toothpast e by
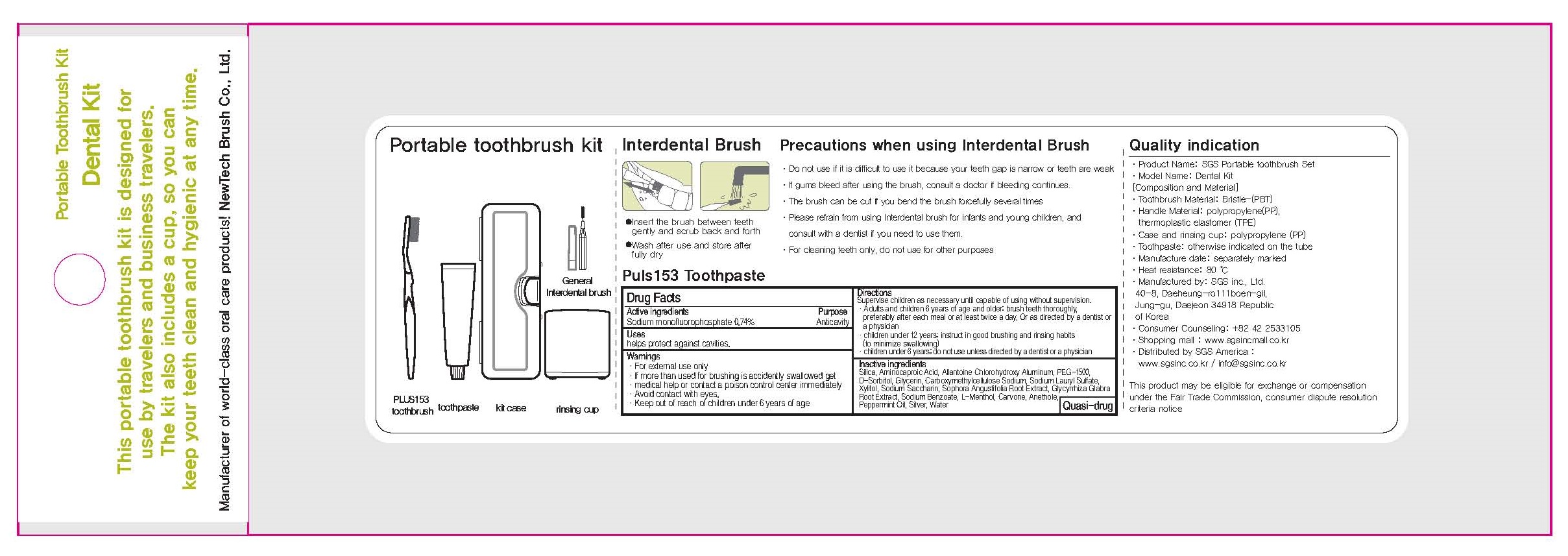
Drug Labeling and Warnings
PLUS153 Toothpast e by is a Otc medication manufactured, distributed, or labeled by SGS Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Inactive ingredients: Silica, Aminocaproic Acid, Allantoine Chlorohydroxy Aluminum, PEG-1500, D-Sorbitol, Glycerin, Carboxymethylcellulose Sodium, Sodium Lauryl Sulfate, Xylitol, Sodium Saccharin, Sophora Angustifolia Root Extract, Glycyrrhiza Glabra Root Extract, Sodium Benzoate, L-Menthol, Carvone, Anethole, Peppermint Oil, Silver, Water
- PURPOSE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- Uses
-
Directions
Directions:
Supervise children as necessary until capable of using without supervision.
- Adults and children 6 years of age and older: brush teeth thoroughly, preferably after each meal or at least twice a day, Or as directed by a dentist or a physician
- children under 12 years: instruct in good brushing and rinsing habits (to minimize swallowing)
- children under 6 years: do not use unless directed by a dentist or a physician
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PLUS153 TOOTHPAST E
sodium monofluorophosphate paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 71553-010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sodium monofluorophosphate (UNII: C810JCZ56Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.29 g in 40 g Inactive Ingredients Ingredient Name Strength Aminocaproic Acid (UNII: U6F3787206) Glycerin (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71553-010-02 1 in 1 POUCH 06/01/2017 1 NDC: 71553-010-01 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part355 06/01/2017 Labeler - SGS Inc. (688296292) Registrant - SGS Inc. (688296292) Establishment Name Address ID/FEI Business Operations SGS Inc. 688296292 manufacture(71553-010)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.