DURISAN HAND SANITIZER- benzalkonium chloride liquid
Durisan by
Drug Labeling and Warnings
Durisan by is a Otc medication manufactured, distributed, or labeled by Sanit Technologies LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredient
- Purpose
- Keep out of reach of children
- Usage
- Warnings
- When Using
- Stop Use
- Dosage
- Inactive Ingredients
-
Durisan Hand Sanitizer 50 ml
Drug Facts
Active Ingredient Purpose
Benzalkonium Chloride 0.1% ………….…. Antiseptic
Uses
- To decrease bacteria on the skin
Warnings
For External use only
When using this product Keep out of eyes. In case of contact, flush eyes with water.
Stop use and consult a doctor if irritation or redness develops.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
- Rub thoroughly over all surfaces of both hands.
- Rub hands together briskly until dry.
Inactive ingredients: water, laurtrimonium chloride, cetrimonium chloride, glycereth-17 cocoate, dihydroxyethyl cocamine oxide, dihydroxypropyl PEG-5, linoeammonium chloride, citric acid
Item # Travel
DHS-0501 Size
Durisan ®
- ANTIMICROBIAL SOLUTIONS
HAND
SANITIZER
ALCOHOL-FREE
LONG-LASTING
NO ODOR
U.S. Patent # 10,058,094
POWERED BY
® SANIT TECHNOLOGIES LLC
50 ml
Net Content 1.69 oz
8 52379 00612 7
Sanit Technologies, LLC
7810 25th Court East Unit 106
Sarasota, Florida 34243
844-DURISAN

-
Durisan Hand Sanitizer 118 ml
Durisan ®
- ANTIMICROBIAL SOLUTIONS
HAND
SANITIZER
ALCOHOL-FREE
LONG-LASTING
NO ODOR
U.S. Patent # 10,058,094
POWERED BY
® SANIT TECHNOLOGIES LLC
118 ml
Net Content 4 oz
Item # DHS-1004
Drug Facts
Active Ingredient Purpose
Benzalkonium Chloride 0.1% ………….…. Antiseptic
Uses
- To decrease bacteria on the skin
Warnings
For External use only
When using this product Keep out of eyes. In case of contact, flush eyes with water.
Stop use and consult a doctor if irritation or redness develops.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
- Rub thoroughly over all surfaces of both hands.
- Rub hands together briskly until dry.
Inactive ingredients: water, laurtrimonium chloride, cetrimonium chloride, glycereth-17 cocoate, dihydroxyethyl cocamine oxide, dihydroxypropyl PEG-5, linoeammonium chloride, citric acid
8 52379 00634 9
Sanit Technologies, LLC
7810 25th Court East Unit 106
Sarasota, Florida 34243
844-DURISAN


-
Durisan 236
Durisan ®
- ANTIMICROBIAL SOLUTIONS
HAND
SANITIZER
ALCOHOL-FREE
LONG-LASTING
NO ODOR
U.S. Patent # 10,058,094
POWERED BY
® SANIT TECHNOLOGIES LLC
236.58ml
Net Content 8 oz
Drug Facts
Active Ingredient Purpose
Benzalkonium Chloride 0.1% ………….…. Antiseptic
Uses
- To decrease bacteria on the skin
Warnings
For External use only
When using this product Keep out of eyes. In case of contact, flush eyes with water.
Stop use and consult a doctor if irritation or redness develops.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
- Rub thoroughly over all surfaces of both hands.
- Rub hands together briskly until dry.
Inactive ingredients: water, laurtrimonium chloride, cetrimonium chloride, glycereth-17 cocoate, dihydroxyethyl cocamine oxide, dihydroxypropyl PEG-5, linoeammonium chloride, citric acid
Item # DHS-1012
8 52379 00635 6
Sanit Technologies, LLC
7810 25th Court East Unit 106
Sarasota, Florida 34243
844-DURISAN
www.DURISAN.com


-
Durisan Hand Sanitizer 250 ml
Drug Facts
Active Ingredient Purpose
Benzalkonium Chloride 0.1% ………….…. Antiseptic
Uses
- To decrease bacteria on the skin
Warnings
For External use only
When using this product Keep out of eyes. In case of contact, flush eyes with water.
Stop use and consult a doctor if irritation or redness develops.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
- Rub thoroughly over all surfaces of both hands.
- Rub hands together briskly until dry.
Inactive ingredients: water, laurtrimonium chloride, cetrimonium chloride, glycereth-17 cocoate, dihydroxyethyl cocamine oxide, dihydroxypropyl PEG-5, linoeammonium chloride, citric acid
Durisan ®
- ANTIMICROBIAL SOLUTIONS
HAND
SANITIZER
ALCOHOL-FREE
LONG-LASTING
NO ODOR
U.S. Patent # 10,058,094
POWERED BY
® SANIT TECHNOLOGIES LLC
250 ml
Net Content 8.45 oz
ITEM # DHS-2501
8 52379 00611 0
Sanit Technologies, LLC
7810 25th Court East
Sarasota, Florida 34243
844-DURISAN
www.DURISAN.com

-
Durisan Hand Sanitizer 300 ml
Durisan ®
- ANTIMICROBIAL SOLUTIONS
ALCOHOL-FREE
HAND SANITIZER
Up to 24 hour protection
Proudly mad in the USA
300 ml
Net Content 10 oz
Drug Facts
Active Ingredient Purpose
Benzalkonium Chloride 0.1% ………….…. Antiseptic
Uses
- To decrease bacteria on the skin
Warnings
For External use only
When using this product Keep out of eyes. In case of contact, flush eyes with water.
Stop use and consult a doctor if irritation or redness develops.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
- Rub thoroughly over all surfaces of both hands.
- Rub hands together briskly until dry.
Inactive ingredients: water, laurtrimonium chloride, cetrimonium chloride, glycereth-17 cocoate, dihydroxyethyl cocamine oxide, dihydroxypropyl PEG-5, linoeammonium chloride, citric acid
Item # RTHS-3000
8 52379 00697 4
Sanit Technologies, LLC
7810 25th Court East
Sarasota, Florida 34243
844-DURISAN
U.S. Patent # 10,058,094

-
Durisan Hand Sanitizer 550 ml
Drug Facts
Active Ingredient Purpose
Benzalkonium Chloride 0.1% ………….…. Antiseptic
Uses
- To decrease bacteria on the skin
Warnings
For External use only
When using this product Keep out of eyes. In case of contact, flush eyes with water.
Stop use and consult a doctor if irritation or redness develops.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
- Rub thoroughly over all surfaces of both hands.
- Rub hands together briskly until dry.
Inactive ingredients: water, laurtrimonium chloride, cetrimonium chloride, glycereth-17 cocoate, dihydroxyethyl cocamine oxide, dihydroxypropyl PEG-5, linoeammonium chloride, citric acid
Durisan ®
- ANTIMICROBIAL SOLUTIONS
HAND
SANITIZER
ALCOHOL-FREE
LONG-LASTING
NO ODOR
U.S. Patent # 10,058,094
POWERED BY
® SANIT TECHNOLOGIES LLC
550 ml
Net Content 18.59 oz
8 52379 00620 2
ITEM # DHS-0550
844-DURISAN
www.DURISAN.com
Sanit Technologies, LLC
7810 25th Court East
Sarasota, Florida 34243

-
Durisan Hand Sanitizer 1000 ml
Durisan ®
- ANTIMICROBIAL SOLUTIONS
HAND
SANITIZER
ALCOHOL-FREE
LONG-LASTING
NO ODOR
U.S. Patent # 10,058,094
POWERED BY
® SANIT TECHNOLOGIES LLC
1000 ml
Net Content 33.81 oz
Drug Facts
Active Ingredient Purpose
Benzalkonium Chloride 0.1% ………….…. Antiseptic
Uses
- To decrease bacteria on the skin
Warnings
For External use only
When using this product Keep out of eyes. In case of contact, flush eyes with water.
Stop use and consult a doctor if irritation or redness develops.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
- Rub thoroughly over all surfaces of both hands.
- Rub hands together briskly until dry.
Inactive ingredients: water, laurtrimonium chloride, cetrimonium chloride, glycereth-17 cocoate, dihydroxyethyl cocamine oxide, dihydroxypropyl PEG-5, linoeammonium chloride, citric acid
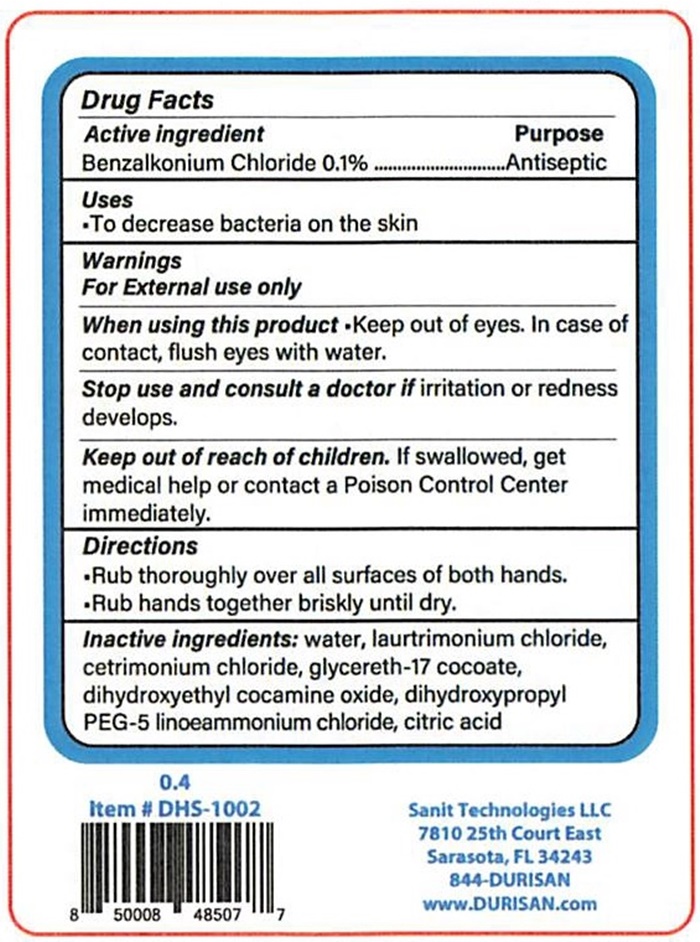
0.4
Item # DHS-1002
8 50008 48507 7
Sanit Technologies, LLC
7810 25th Court East
Sarasota, Florida 34243
844-DURISAN
www.DURISAN.com
Drug Facts
Active Ingredient Purpose
Benzalkonium Chloride 0.1% ………….…. Antiseptic
Uses
- To decrease bacteria on the skin
Warnings
For External use only
When using this product Keep out of eyes. In case of contact, flush eyes with water.
Stop use and consult a doctor if irritation or redness develops.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
- Rub thoroughly over all surfaces of both hands.
- Rub hands together briskly until dry.
Inactive ingredients: water, laurtrimonium chloride, cetrimonium chloride, glycereth-17 cocoate, dihydroxyethyl cocamine oxide, dihydroxypropyl PEG-5, linoeammonium chloride, citric acid
0.8
Item # DHS-1008
8 52379 00610 3
Sanit Technologies, LLC
7810 25th Court East
Sarasota, Florida 34243
844-DURISAN
www.DURISAN.COM


-
Durisan Hand Sanitizer 1 Gallon
Sanit Technologies, LLC
7810 25th Court East
Sarasota, Florida 34243
844-DURISAN
www.DURISAN.com
ITEM # DHS-3785
8 52379 00621 9
Drug Facts
Active Ingredient Purpose
Benzalkonium Chloride 0.1% ………….…. Antiseptic
Uses
- To decrease bacteria on the skin
Warnings
For External use only
When using this product Keep out of eyes. In case of contact, flush eyes with water.
Stop use and consult a doctor if irritation or redness develops.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
- Rub thoroughly over all surfaces of both hands.
- Rub hands together briskly until dry.
Inactive ingredients: water, laurtrimonium chloride, cetrimonium chloride, glycereth-17 cocoate, dihydroxyethyl cocamine oxide, dihydroxypropyl PEG-5, linoeammonium chloride, citric acid
Durisan ®
- ANTIMICROBIAL SOLUTIONS
HAND
SANITIZER
ALCOHOL-FREE
LONG-LASTING
NO ODOR
U.S. Patent # 10,058,094
POWERED BY
® SANIT TECHNOLOGIES LLC
1 Gallon
Net Content 128 oz

-
INGREDIENTS AND APPEARANCE
DURISAN HAND SANITIZER
benzalkonium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 71120-611 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 2.5 mg in 250 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIHYDROXYPROPYL PEG-5 LINOLEAMMONIUM CHLORIDE (UNII: 0Y0NQR2GH1) GLYCERETH-2 COCOATE (UNII: JWM00VS7HC) BEHENTRIMONIUM CHLORIDE (UNII: X7GNG3S47T) DIHYDROXYETHYL COCAMINE OXIDE (UNII: 8AR51R3BL5) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71120-611-01 20 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/01/2016 02/28/2020 2 NDC: 71120-611-02 50 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/01/2016 3 NDC: 71120-611-03 250 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/01/2016 4 NDC: 71120-611-04 550 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/01/2016 5 NDC: 71120-611-05 3785 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/01/2016 6 NDC: 71120-611-06 1000 mL in 1 CONTAINER; Type 0: Not a Combination Product 01/01/2016 7 NDC: 71120-611-07 118 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 01/01/2016 8 NDC: 71120-611-08 237 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 01/01/2016 9 NDC: 71120-611-09 4 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 01/01/2016 02/28/2020 10 NDC: 71120-611-10 8 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 01/01/2016 02/28/2020 11 NDC: 71120-611-11 300 mL in 1 CONTAINER; Type 0: Not a Combination Product 01/01/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 01/01/2016 Labeler - Sanit Technologies LLC (075711022) Establishment Name Address ID/FEI Business Operations Lab Express International 800875106 relabel(71120-611) , api manufacture(71120-611) , repack(71120-611) Establishment Name Address ID/FEI Business Operations Organics Corporation of America DBA Ambix Laboratories 061061164 manufacture(71120-611) Establishment Name Address ID/FEI Business Operations Durisan 085479946 manufacture(71120-611)
Trademark Results [Durisan]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DURISAN 88260470 not registered Live/Pending |
Sanit Technologies LLC 2019-01-14 |
 DURISAN 88260429 not registered Live/Pending |
Sanit Technologies LLC 2019-01-14 |
 DURISAN 86626494 not registered Dead/Abandoned |
Sanit Technologies, LLC 2015-05-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.