FEXOFENADINE HYDROCHLORIDE AND PSEUDOEPHEDRINE HYDROCHLORIDE- fexofenadine hydrochloride and pseudoephedrine hydrochloride tablet, film coated, extended release
Fexofenadine Hydrochloride and Pseudoephedrine Hydrochloride by
Drug Labeling and Warnings
Fexofenadine Hydrochloride and Pseudoephedrine Hydrochloride by is a Otc medication manufactured, distributed, or labeled by AmeriSource Bergen, Sun Pharmaceutical Industries Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
- temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
- reduces swelling of nasal passages
- temporarily relieves sinus congestion and pressure
- temporarily restores freer breathing through the nose
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
-
Warnings
Do not use
- if you have ever had an allergic reaction to this product or any of its ingredients
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- if you have difficulty swallowing
Ask a doctor before use if you have
- heart disease
- thyroid disease
- glaucoma
- high blood pressure
- diabetes
- trouble urinating due to an enlarged prostate gland
- kidney disease. Your doctor should determine if you need a different dose.
When using this product
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
-
Directions
- do not divide, crush, chew or dissolve the tablet; swallow tablet whole
adults and children 12 years of age and over take 1 tablet with a glass of water every 12 hours on an empty stomach; do not take more than 2 tablets in 24 hours children under 12 years of age do not use adults 65 years of age and older ask a doctor consumers with kidney disease ask a doctor - Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
-
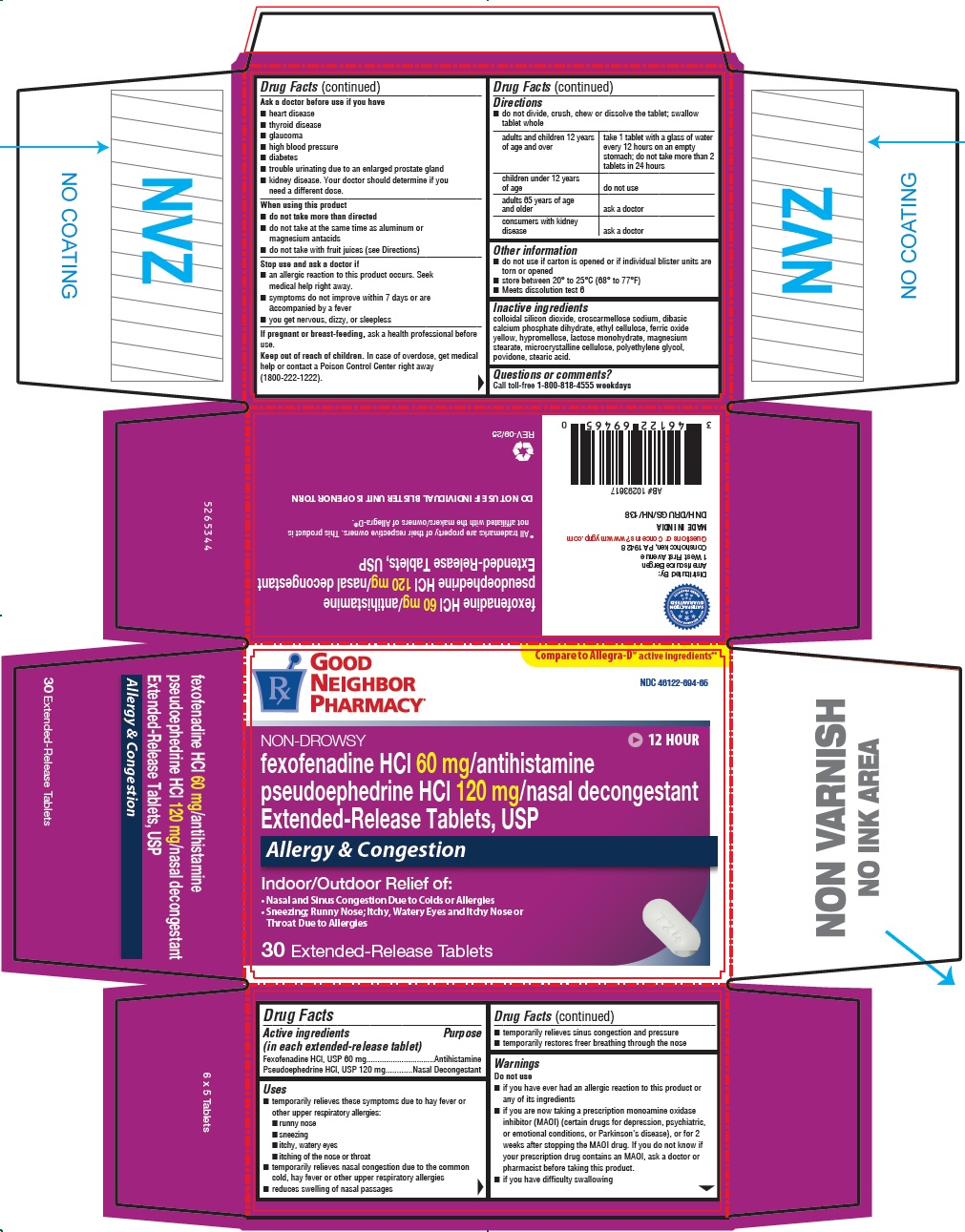
PRINCIPAL DISPLAY PANEL - 30 Tablet Blister Pack Carton
Compare to Allegra-D ®active ingredients**
GOOD
NEIGHBOR
PHARMACY ®NDC: 46122-694-65
NON-DROWSY
12 HOUR
fexofenadine HCl 60 mg/antihistamine
pseudoephedrine HCl 120 mg/nasal decongestant
Extended-Release Tablets, USPIndoor & Outdoor Allergies
Allergy & Congestion
Indoor/Outdoor Relief of:
- Nasal and Sinus Congestion Due to Colds or Allergies
- Sneezing; Runny Nose; Itchy, Watery Eyes and Itchy Nose or
Throat Due to Allergies
30 Extended-Release Tablets
-
INGREDIENTS AND APPEARANCE
FEXOFENADINE HYDROCHLORIDE AND PSEUDOEPHEDRINE HYDROCHLORIDE
fexofenadine hydrochloride and pseudoephedrine hydrochloride tablet, film coated, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 46122-694 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 60 mg PSEUDOEPHEDRINE HYDROCHLORIDE (UNII: 6V9V2RYJ8N) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE HYDROCHLORIDE 120 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) POVIDONE K30 (UNII: U725QWY32X) MAGNESIUM STEARATE (UNII: 70097M6I30) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ETHYLCELLULOSE, UNSPECIFIED (UNII: 7Z8S9VYZ4B) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) STEARIC ACID (UNII: 4ELV7Z65AP) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Product Characteristics Color white, yellow Score no score Shape CAPSULE (bilayer) Size 17mm Flavor Imprint Code 724 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 46122-694-65 3 in 1 CARTON 03/01/2018 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090818 03/01/2018 Labeler - AmeriSource Bergen (007914906) Establishment Name Address ID/FEI Business Operations Sun Pharmaceutical Industries Limited 650445203 analysis(46122-694) , manufacture(46122-694)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.