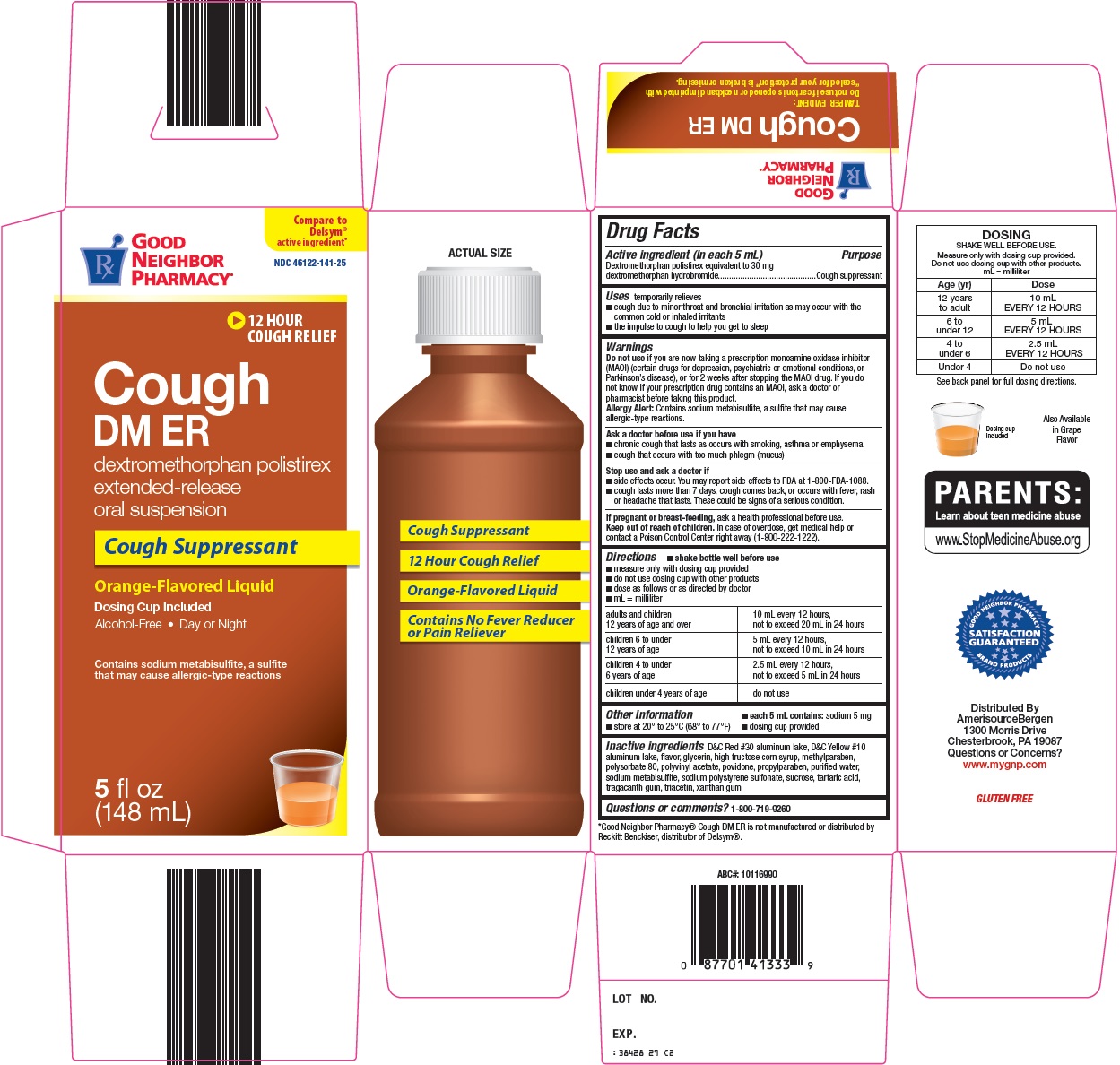
GOOD NEIGHBOR PHARMACY COUGH DM ER- dextromethorphan polistirex suspension
Good Neighbor Pharmacy Cough DM ER by
Drug Labeling and Warnings
Good Neighbor Pharmacy Cough DM ER by is a Otc medication manufactured, distributed, or labeled by Amerisource Bergen. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (in each 5 mL)
- Purpose
- Uses
-
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Allergy Alert: Contains sodium metabisulfite, a sulfite that may cause allergic-type reactions.
Ask a doctor before use if you have
- chronic cough that lasts as occurs with smoking, asthma or emphysema
- cough that occurs with too much phlegm (mucus)
- Keep out of reach of children.
-
Directions
- shake bottle well before use
- measure only with dosing cup provided
- do not use dosing cup with other products
- dose as follows or as directed by doctor
- mL = milliliter
adults and children 12 years of age and over
10 mL every 12 hours, not to exceed 20 mL in 24 hours
children 6 to under 12 years of age
5 mL every 12 hours, not to exceed 10 mL in 24 hours
children 4 to under 6 years of age
2.5 mL every 12 hours, not to exceed 5 mL in 24 hours
children under 4 years of age
do not use
- Other information
-
Inactive Ingredients
D&C Red #30 aluminum lake, D&C Yellow #10 aluminum lake, flavor, glycerin, high fructose corn syrup, methylparaben, polysorbate 80, polyvinyl acetate, povidone, propylparaben, purified water, sodium metabisulfite, sodium polystyrene sulfonate, sucrose, tartaric acid, tragacanth gum, triacetin, xanthan gum
- Questions or comments?
-
Package/Label Principal Display Panel
Compare to Delsym® active ingredient
12 HOUR COUGH RELIEF
Cough
DM ER
dextromethorphan polistirex
extended-release oral suspension
Cough Suppressant
Orange-Flavored Liquid
Dosing Cup Included
Alcohol-Free – Day or Night
Contains sodium metabisulfite, a sulfite that may cause allergic-type reactions
5 fl oz (148 mL)
-
INGREDIENTS AND APPEARANCE
GOOD NEIGHBOR PHARMACY COUGH DM ER
dextromethorphan polistirex suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 46122-141 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 30 mg in 5 mL Inactive Ingredients Ingredient Name Strength POLISTIREX (UNII: 5H9W9GTW27) D&C RED NO. 30 (UNII: 2S42T2808B) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) GLYCERIN (UNII: PDC6A3C0OX) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) METHYLPARABEN (UNII: A2I8C7HI9T) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POLYVINYL ACETATE (UNII: 32K497ZK2U) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SODIUM METABISULFITE (UNII: 4VON5FNS3C) SODIUM POLYSTYRENE SULFONATE (UNII: 1699G8679Z) SUCROSE (UNII: C151H8M554) TARTARIC ACID (UNII: W4888I119H) TRAGACANTH (UNII: 2944357O2O) TRIACETIN (UNII: XHX3C3X673) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color ORANGE Score Shape Size Flavor ORANGE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 46122-141-21 1 in 1 CARTON 08/30/2012 1 89 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 46122-141-25 1 in 1 CARTON 08/02/2013 2 148 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091135 08/30/2012 Labeler - Amerisource Bergen (007914906)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.