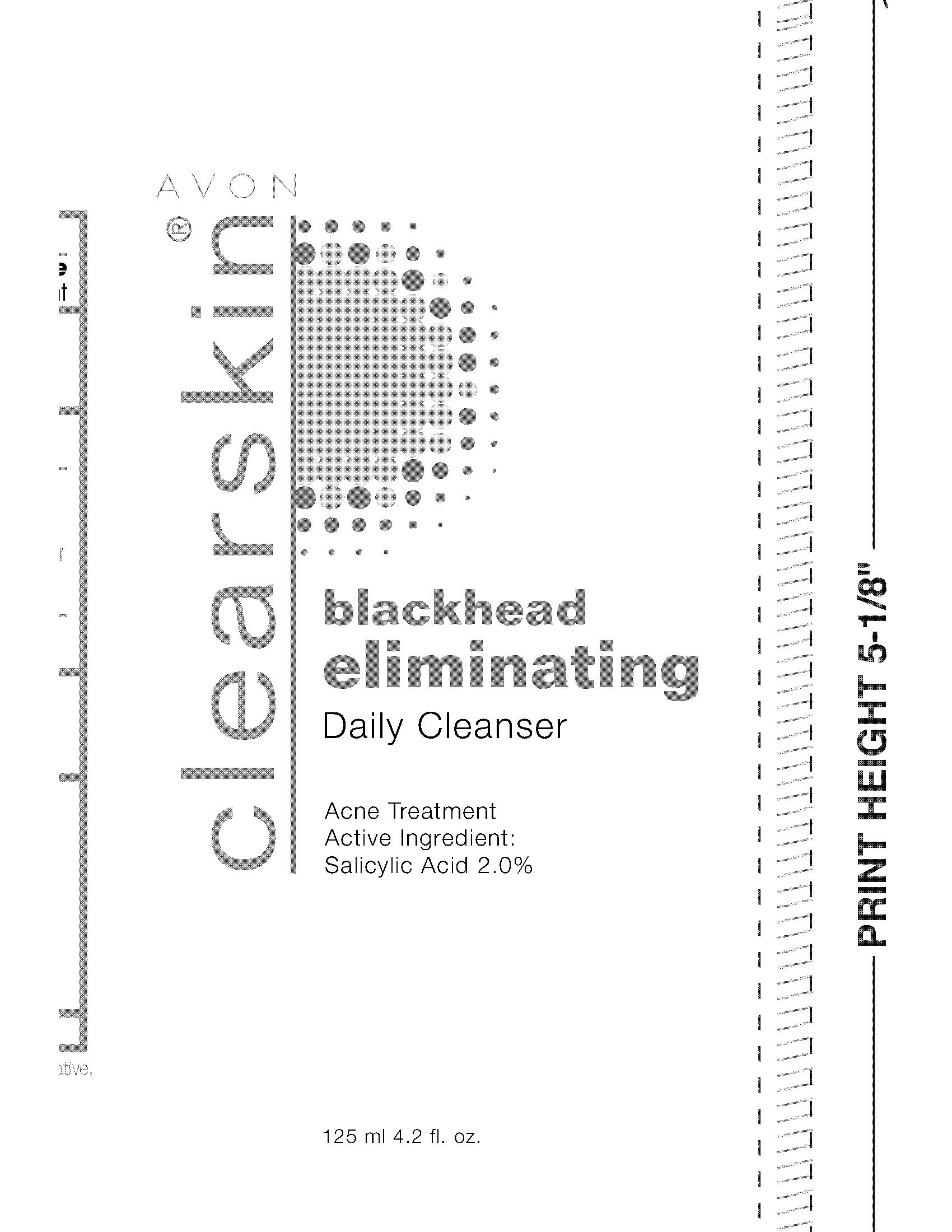
CLEARSKIN BLACKHEAD ELIMINATING CLEANSER- salicylic acid gel
Clearskin by
Drug Labeling and Warnings
Clearskin by is a Otc medication manufactured, distributed, or labeled by New Avon LLC, Fareva Morton Grove, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use onlyWhen using this product
- avoid contact with eyes. If product contacts eyes, rinse thoroughly with water. If irritation persists, seek medical attention.
- using other topical acne medications at the same time or immediately after use of this product may increase dryness or irritation of the skin. If this occurs, only one medication should be used unless directed by a doctor.
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Inactive ingredients Water/Eau, Sodium C14-16 Olefin Sulfonate,
Glycerin, Cocamidopropyl Betain, Polyethylene, Sodium Methyl Cocoyl
Taurate, Acrylates Copolymer, Sodium Chloride, Aluminum Starch
Octenylsuccinate, Sodium Lauroamphoacetate, Aloe Barbadensis Leaf
Juice, Chamomilla Recutita (Matricaria) Flower Extract, Citric Acid, C20-40
Alcohols, Parfum/Fragrance, Cocamidopropyl PG-Dimonium Chloride
Phosphate, Disodium EDTA, Isododecane, Lysine Carboxymethyl Cysteinate,
Polystyrene/Hydrogenated Polyisopentene Copolymer, Sodium C8-16
Isoalkylsuccinyl Lactoglobulin Sulfonate, Sodium Hydroxide, Blue 1
Lake/CI 42090, Violet 2/CI 60725. - QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CLEARSKIN BLACKHEAD ELIMINATING CLEANSER
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 10096-0182 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10096-0182-1 125 mL in 1 TUBE; Type 0: Not a Combination Product 12/16/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 12/16/2009 Labeler - New Avon LLC (080143520) Establishment Name Address ID/FEI Business Operations Fareva Morton Grove, Inc. 116752326 manufacture(10096-0182)
Trademark Results [Clearskin]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CLEARSKIN 97653073 not registered Live/Pending |
Alma Lasers Ltd 2022-10-28 |
 CLEARSKIN 77745124 3707213 Dead/Cancelled |
Anthropics Technology Ltd 2009-05-27 |
 CLEARSKIN 77644093 not registered Dead/Abandoned |
DAYMEN PHOTO MARKETING LP 2009-01-06 |
 CLEARSKIN 74687583 1976573 Live/Registered |
AVON NA IP LLC 1995-06-12 |
 CLEARSKIN 74687433 not registered Dead/Abandoned |
Avon Products, Inc. 1995-06-12 |
 CLEARSKIN 74515428 1975701 Live/Registered |
AVON NA IP LLC 1994-04-22 |
 CLEARSKIN 74515427 not registered Dead/Abandoned |
Avon Products, Inc. 1994-04-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.