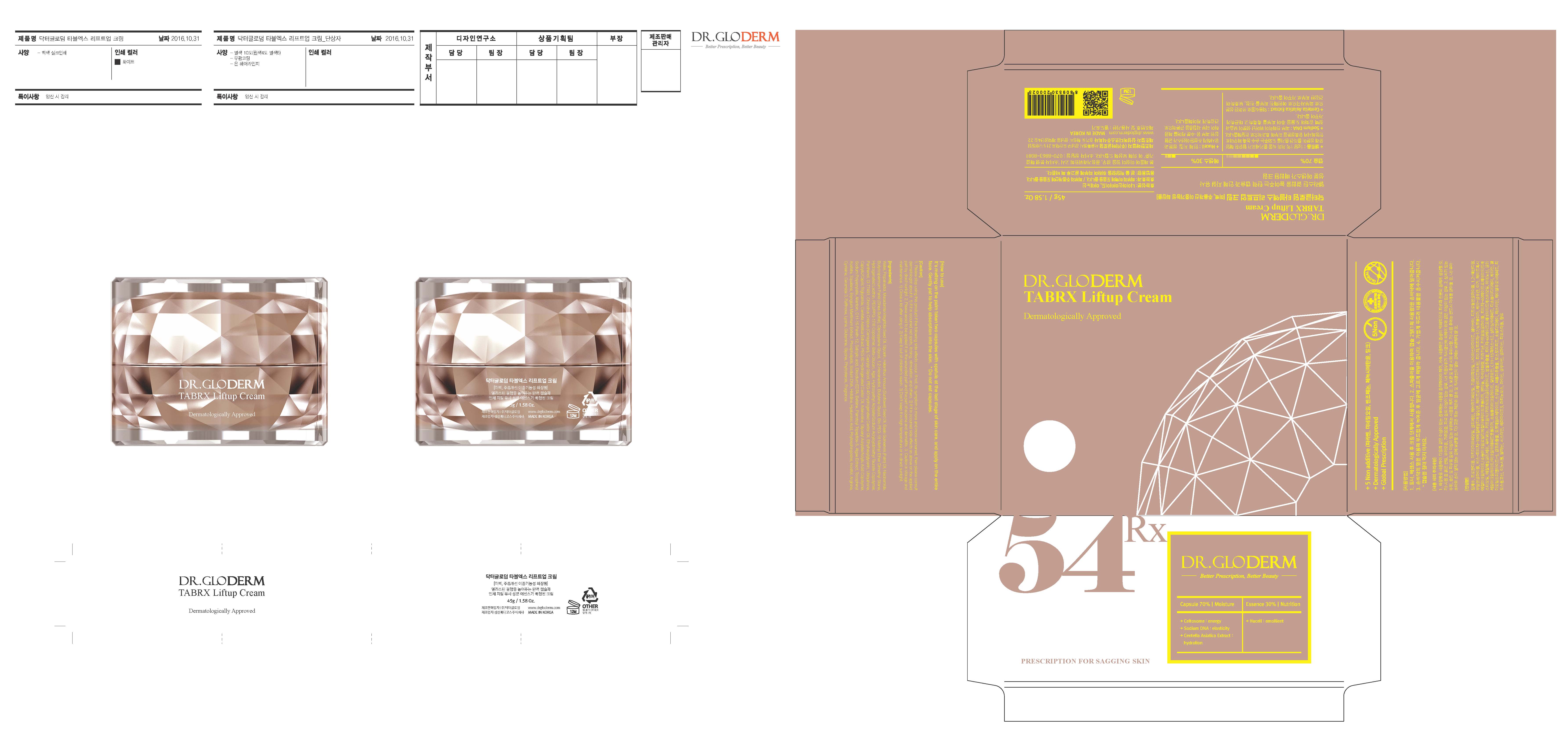
DR. GLODERM TABRX LIFTUP- niacinamide, adenosine cream
DR. GLODERM TABRX LIFTUP by
Drug Labeling and Warnings
DR. GLODERM TABRX LIFTUP by is a Otc medication manufactured, distributed, or labeled by DR.GLODERM, SAMSUNG MEDICOS. CO., LTD. Hyangnam Factory. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
1. If the following symptoms occur after product use, stop using the product immediately and consult a dermatologist (continuous use can exacerbate the symptoms).
1) Occurrence of red spots, swelling, itchiness, and other skin irritation
2) If the symptoms above occur after the application area is exposed to direct sunlight
2. Do not use on open wounds, eczema, and other skin irritations
3. Precaution for Storage and Handling
1) Close the lid after use
2) Keep out of reach of infants and children
3) Do not to store in a place with high/low temperature and exposed to direct sunlight4. Use as avoiding eye areas.
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DR. GLODERM TABRX LIFTUP
niacinamide, adenosine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 71342-0010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ADENOSINE (UNII: K72T3FS567) (ADENOSINE - UNII:K72T3FS567) ADENOSINE 0.04 g in 100 g NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 2.14 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71342-0010-1 1 in 1 PACKAGE 03/01/2017 03/28/2017 1 1 in 1 APPLICATOR 1 45 g in 1 JAR; Type 0: Not a Combination Product 2 NDC: 71342-0010-2 45 g in 1 JAR; Type 0: Not a Combination Product 03/28/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/01/2017 Labeler - DR.GLODERM (694773267) Registrant - DR.GLODERM (694773267) Establishment Name Address ID/FEI Business Operations SAMSUNG MEDICOS. CO., LTD. Hyangnam Factory 689851701 manufacture(71342-0010) Establishment Name Address ID/FEI Business Operations DR.GLODERM 694773267 label(71342-0010)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.