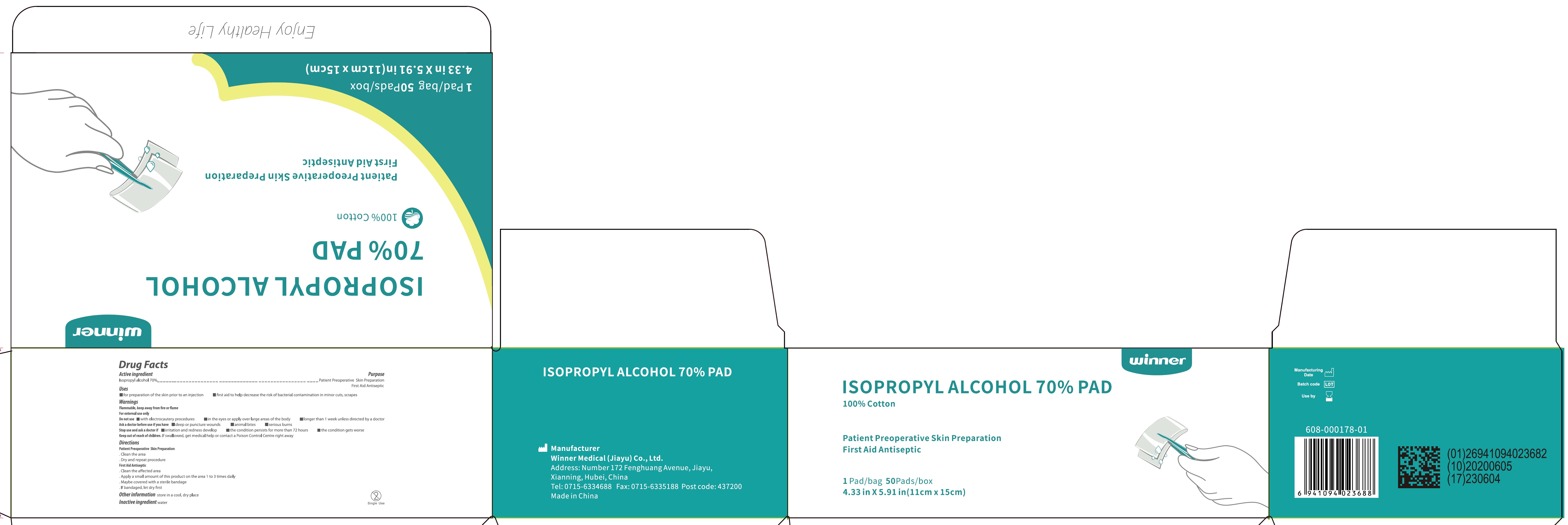
Isopropyl Alcohol 70% Pad
Isopropyl Alcohol 70 Pad by
Drug Labeling and Warnings
Isopropyl Alcohol 70 Pad by is a Otc medication manufactured, distributed, or labeled by Winner Medical (Jiayu) Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ISOPROPYL ALCOHOL 70 PAD- isopropyl alcohol swab
Winner Medical (Jiayu) Co., Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Isopropyl Alcohol 70% Pad
Uses
- for preparation of the skin prior to an injection
- first aid to help decrease the risk of bacteria contamination in minor cuts, scrapes
Warnings
Flammable, keep away from fire or flame
For external use only
Do not use
- with electrocautery procedures
- in the eyes or apply over large areas of the body
- longer that 1 week unless directed by a doctor
| ISOPROPYL ALCOHOL 70 PAD
isopropyl alcohol swab |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Winner Medical (Jiayu) Co., Ltd. (530058569) |
Revised: 10/2021
Document Id: cea404b9-eb18-d35b-e053-2995a90a5b45
Set id: 4b78ab42-3a5e-4b33-bef8-47acaa097807
Version: 2
Effective Time: 20211018