PURALUBE- light mineral oil, white petrolatum ointment
Puralube by
Drug Labeling and Warnings
Puralube by is a Otc medication manufactured, distributed, or labeled by Paddock Laboratories, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredients
- Purpose
- Uses
- Warnings
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
- Directions
- Other information
- Questions or comments?
-
Consumer information
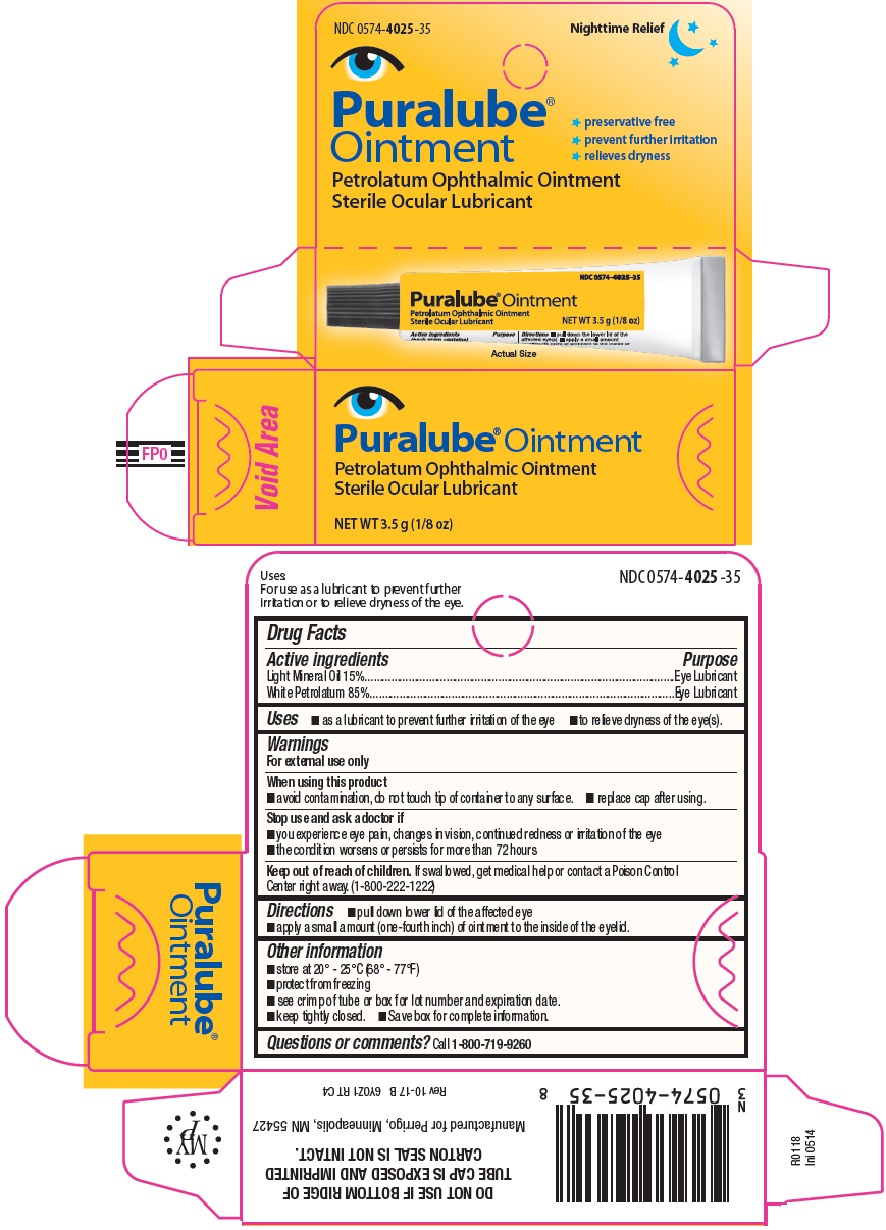
PURALUBE® OINTMENT
PETROLATUM OPHTHALMIC OINTMENT
STERILE
OCULAR LUBRICANT
DESCRIPTION: a sterile ocular emollient (lubricant).
Active ingredients
Light mineral oil 15%
White petrolatum 85%
Purposes
Eye lubricant
Uses: for use as a lubricant to prevent further irritation or to relieve dryness of the eye.
Warnings:
For external use only
When using this product:
- To avoid contamination of this product do not touch the tip of the container to any surface.
- For the multi-use container: Replace the cap after using.
- For the single-use container: Do not reuse. Once opened, discard.
Stop using and ask a doctor if:
- You experience eye pain.
- Changes in vision.
- Continued redness or irritation of the eye.
- The condition worsens or persists for more than 72 hours.
Directions: pull down the lower lid of the affected eye and apply a small amount (one-fourth inch) of ointment to the inside of the eyelid.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
DO NOT USE IF BOTTOM RIDGE OF TUBE CAP IS EXPOSED.
See crimp of tube or box for lot number and expiration date.
Store at 20 to 25°C (68 to 77°F)
KEEP TIGHTLY CLOSED
HOW SUPPLIED:
1/8 OZ (3.5 g) Tube (multi-use container) NDC 0574-4025-35
Carton of Twenty (20) Unit Dose 1 g Tube (single-use container) NDC 0574-4025-20
Manufactured for Perrigo, Minneapolis, MN 55427
Questions or comments? Call 1-800-719-9260
Rev 04-14 A
6Y000 RT J1
R0414
Ini 0414
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
PURALUBE
light mineral oil, white petrolatum ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0574-4025 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIGHT MINERAL OIL (UNII: N6K5787QVP) (LIGHT MINERAL OIL - UNII:N6K5787QVP) LIGHT MINERAL OIL 150 mg in 1 g PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 850 mg in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0574-4025-35 1 in 1 CARTON 01/05/2015 01/01/2022 1 3.5 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC: 0574-4025-20 20 in 1 CARTON 07/29/2014 11/01/2020 2 NDC: 0574-4025-11 1 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 07/28/2014 01/01/2022 Labeler - Paddock Laboratories, LLC (967694121)
Trademark Results [Puralube]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PURALUBE 75150615 not registered Dead/Abandoned |
Puralube, Inc. 1996-08-15 |
 PURALUBE 74649126 not registered Dead/Abandoned |
Puralube, Inc. 1995-03-20 |
 PURALUBE 74032619 1620792 Live/Registered |
Altana Inc. 1990-02-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.