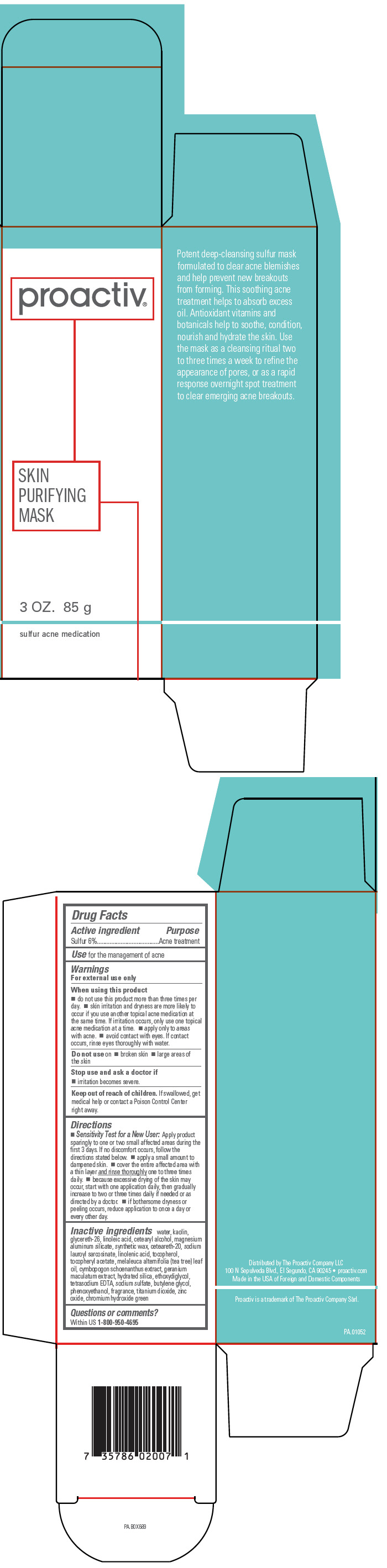
Proactiv® Skin Purifying Mask
Proactiv Skin Purifying Mask by
Drug Labeling and Warnings
Proactiv Skin Purifying Mask by is a Otc medication manufactured, distributed, or labeled by The Proactiv Company, LLC, VEE PAK, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PROACTIV SKIN PURIFYING MASK- sulfur lotion
The Proactiv Company, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Proactiv® Skin Purifying Mask
Warnings
For external use only
When using this product
- do not use this product more than three times per day.
- skin irritation and dryness are more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- apply only to areas with acne.
- avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Directions
- Sensitivity Test for a New User: Apply product sparingly to one or two small affected areas during the first 3 days. If no discomfort occurs, follow the directions stated below.
- apply a small amount to dampened skin.
- cover the entire affected area with a thin layer and rinse thoroughly one to three times daily.
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor.
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day.
Inactive ingredients
water, kaolin, glycereth-26, linoleic acid, cetearyl alcohol, magnesium aluminum silicate, synthetic wax, ceteareth-20, sodium lauroyl sarcosinate, linolenic acid, tocopherol, tocopheryl acetate, melaleuca alternifolia (tea tree) leaf oil, cymbopogon schoenanthus extract, geranium maculatum extract, hydrated silica, ethoxydiglycol, tetrasodium EDTA, sodium sulfate, butylene glycol, phenoxyethanol, fragrance, titanium dioxide, zinc oxide, chromium hydroxide green
| PROACTIV SKIN PURIFYING MASK
sulfur lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - The Proactiv Company, LLC (080216357) |
| Registrant - VEE PAK, LLC (874763303) |