Calcium Edetate de Sodium by BTG International Inc
Calcium Edetate de Sodium by
Drug Labeling and Warnings
Calcium Edetate de Sodium by is a Prescription medication manufactured, distributed, or labeled by BTG International Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CALCIUM EDETATE DE SODIUM- edetate calcium disodium anhydrous solution
BTG International Inc
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
HEALTHCARE PROVIDER LETTER
IMPORTANT PRESCRIBING INFORMATION
21 October 2022
Subject: Temporary Importation of SERB’s Calcium Edetate de Sodium Injection to Address Drug Shortage
Dear Healthcare Professional:
Due to current critical shortage of Calcium Disodium Versenate Injection in the United States (US) market, BTG International Inc. (BTG), in conjunction with SERB SAS (SERB), is coordinating with the US Food and Drug Administration (FDA) to temporarily import non-FDA approved Calcium Edetate de Sodium Injection (500 mg/ampule, 5 g/carton) that is authorized for marketing in France. The FDA has not approved SERB’s Calcium Edetate de Sodium Injection in the US. Calcium Edetate de Sodium is indicated for the treatment of lead poisoning.
At this time, BTG and/or its distributor, ASD Healthcare, are the only US distributors of the imported Calcium Edetate de Sodium Injection.
Effective immediately, BTG will import and distribute the following presentation of SERB’s Calcium Edetate de Sodium injection to address the critical shortage:
| Product Name | Strength | Dosage Form | Package Size | Lot Number | NDC Number |
| Calcium Edetate de Sodium Injection | 500 mg/ampule | Injection, Solution (in ampules) | 10 ampules | 3081 | 50633-320-10 |
Packaging Information:
The ampule and carton labels will display the text intended for marketing in France with both English and French translations. It is important to note that there are differences in the format and content of the labelling between Calcium Disodium Versenate Injection and SERB’s Calcium Edetate de Sodium Injection. The imported product has a concentration of 500 mg/10ml 50 mg/ml, while the FDA approved US version has a concentration of 200 mg/mL. Both products are contraindicated for patients with kidney disease. The imported product is contraindicated for patients with an allergy against calcium edetate and also in combination with digitalis. The FDA approved US version is contraindicated against patients with hepatitis or have had periods of anuria. See the table on page 2 for identification of the differences.
The barcode on the imported product label will not register accurately on US scanning systems. Alternative procedures should be followed to assure that the correct drug product is being used and administered to individual patients. In addition, the packaging of the imported product does not include serialization information. SERB’s Calcium Edetate de Sodium Injection does not meet US Drug Supply Chain Security Act (DSCSA) requirements
SERB’s Calcium Edetate de Sodium Injection is available only by prescription in the US. However, the imported lot does not have the statement “Rx only” on their labelling. Please refer to the package insert for full prescribing information. This, and additional product comparison information, can be found on BTG’s website https://serb.com/edta_us
To order: Please contact:
| ASD Healthcare |
| Phone: 800.746.6273 / Fax: 800.547.9413 / Email: service@asdhealthcare.com |
Please ensure that your staff and others in your institution who may be involved in the administration of Calcium Edetate de Sodium Injection receive a copy of this letter, review the information and maintain a copy of this letter with the product at all times.
Adverse Event Reporting: Healthcare providers should report adverse events associated with the use of SERB’s Calcium Edetate de Sodium Injection to BTG at 844-293-0007. Adverse events or quality problems experienced with the use of this product may also be reported to the FDA’s MedWatch Adverse Event Reporting Program either online, by regular mail, or by fax:
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular mail or Fax: Download form
https://www.accessdata.fda.gov/scripts/medwatch/index.cfm?action=reporting.home - or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the preaddressed form or submit by fax to 1-800-FDA-0178 (1-800-332-0178).
Questions/Support: If you have any questions about the information contained in this letter, any quality related problems, or questions on the use of SERB’s Calcium Edetate de Sodium Injection, please contact BTG’s 24-hour line at 877-377-3784 or send an email to EDTA@btgsp.com. We remain at your disposal to answer any questions you may have about our product; and provide more information if needed.
Sincerely,

Christon Hill
Chief Innovation Officer
| Import Product | US FDA Approved Product | |
| Product Name | Calcium Edetate de Sodium Injection | Calcium Disodium Versenate Injection |
| Amount per Carton | 5000 mg (5 grams) | 5000 mg (5 grams) |
| Amount per Ampule | 500 mg | 1000 mg |
| Concentration | 50 mg/mL | 200 mg/mL |
| Volume | 10 mL per ampule | 5 mL per ampule |
| Units per Carton | 10 ampules | 5 ampules |
| Indication | This medicinal product is indicated for the treatment of lead poisoning. | For the reduction of blood levels and depot stores of lead in lead poisoning (acute and chronic) and lead encephalopathy, in both pediatric populations and adults. Chelation therapy should not replace effective measures to eliminate or reduce further exposure to lead. |
| Dosing | Acute situations: 1 to 2 ampoules per day, usually for 5 days. After a rest period of 7 days, start another treatment course for 5 days, with 1 to 2 ampoules per day. | When a source for the lead intoxication has been identified, the patient should be removed from the source, if possible. The recommended dose of Calcium Disodium Versenate for asymptomatic adults and pediatric patients whose blood lead level is < 70 mcg/dL but > 20 mcg/dL is 1,000 mg/m2/day whether given intravenously or intramuscularly. For adults with lead nephropathy, the following dosing regimen has been suggested: 500 mg/m2 every 24 hours for 5 days for patients with serum creatinine levels of 2-3 mg/dL, every 48 hours for 3 doses for patients with creatinine levels of 3-4 mg/dL, and once weekly for patients with creatinine levels above 4 mg/dL. These regimens may be repeated at one-month intervals. Calcium Disodium Versenate, used alone, may aggravate symptoms in patients with very high blood lead levels. When the blood lead level is > 70 mcg/dL or clinical symptoms consistent with lead poisoning are present, it is recommended that Calcium Disodium Versenate be used in conjunction with BAL (dimercaprol). Please consult published protocols and specialized references for dosage recommendations of combination therapy. Therapy of lead poisoning in adults and pediatric patients with Calcium Disodium Versenate is continued over a period of five days. Therapy is then interrupted for 2 to 4 days to allow redistribution of the lead and to prevent severe depletion of zinc and other essential metals. Two courses of treatment are usually employed; however, it depends on severity of the lead toxicity and the patient’s tolerance of the drug. Calcium Disodium Versenate is equally effective whether administered intravenously or intramuscularly. The intramuscular route is used for all patients with overt lead encephalopathy and this route is preferred by some for young pediatric patients. Acutely ill individuals may be dehydrated from vomiting. Since edetate calcium disodium is excreted almost exclusively in the urine, it is very important to establish urine flow with intravenous fluids administration before the first dose of the chelating agent is given; however, excessive fluid must be avoided in patients with encephalopathy. Once urine flow is established, further intravenous fluid is restricted to basal water and electrolyte requirements. Administration of Calcium Disodium Versenate should be stopped whenever there is cessation of urine flow in order to avoid unduly high tissue levels of the drug. Edetate calcium disodium must be used in reduced doses in patients with pre- existing mild renal disease. |
| Methods of Administration | Intravenous route: slow intravenous infusion. The content of each ampoule must be administered by slow intravenous infusion, diluted in 250 ml of isotonic saline or glucose solution. | Intravenous Administration
Add the total daily dose of Calcium Disodium Versenate (1000 mg/m2/day) to 250-500 ml of 5% dextrose or 0.9% sodium chloride injection. The total daily dose should be infused over a period of 8-12 hours. Calcium Disodium Versenate injection is incompatible with 10% dextrose, 10% invert sugar in 0.9% sodium chloride, lactate Ringer’s, Ringer’s, one-sixth molar sodium lactate injections, and with injectable amphotericin B and hydralazine hydrochloride. Intramuscular Administration The total daily dosage (1000 mg/m2/day) should be divided into equal doses spaced 8-12 hours apart. Lidocaine or procaine should be added to the Calcium Disodium Versenate injection to minimize pain at the injection site. The final lidocaine or procaine concentration of 5 mg/ml (0.5%) can be obtained as follows: 0.25 ml of 10% lidocaine solution per 5 ml concentrated Calcium Disodium Versenate; 1 ml of 1% lidocaine or procaine solution per ml of concentrated Calcium Disodium Versenate. When used alone, regardless of method of administration, Calcium Disodium Versenate should not be given at doses larger than those recommended. |
| Contraindications | Do not use CALCIUM EDETATE DE SODIUM SERB 50 mg / ml, solution for I.V. injection: - if you are allergic to sodium calcium edetate or any of the other ingredients of this medicine (listed in section 6), - if you have anuri disease, - in combination with digitalis. | Edetate calcium disodium should not be given during periods of anuria, nor to patients with active renal disease or hepatitis. |
| Boxed Warning | No boxed warning. | WARNINGS: Calcium Disodium Versenate is capable of producing toxic effects which can be fatal. Lead encephalopathy is relatively rare in adults, but occurs more often in pediatric patients in whom it may be incipient and thus overlooked. The mortality rate in pediatric patients has been high. Patients with lead encephalopathy and cerebral edema may experience a lethal increase in intracranial pressure following intravenous infusion; the intramuscular route is preferred for these patients. In cases where the intravenous route is necessary, avoid rapid infusion. The dosage schedule should be followed and at no time should the recommended daily dose be exceeded. |
| Warnings and Precautions | Warnings and precautions Talk to your doctor or pharmacist before using CALCIUM EDETATE DE SODIUM SERB 50 mg / ml, solution for I.V. injection. In case of kidney disease, inform your doctor. This medicine must be injected as a slow infusion. Other medicines and CALCIUM EDETATE DE SODIUM SERB 50 mg / ml, solution for I.V. injection: Inform your doctor if you take digitalis (medicines used in some cardiac diseases). Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. CALCIUM EDETATE DE SODIUM SERB 50 mg / ml, solution for I.V. injection contains sodium. This medicine contains 61.5 mg sodium (main component of cooking/table salt) in each ampoule. This is equivalent to 3% of the recommended maximum daily dietary intake of sodium for an adult. Biological tests (blood samples) may be performed before starting and during the treatment in order to monitor your renal function. Pregnancy, breast-feeding and fertility If needed, this medicine can be used during pregnancy. Breast-feeding should be avoided during the use of this medicine. If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor orpharmacist for advice before taking this medicine. | General Precautions: Edetate calcium disodium may produce the same renal damage as lead poisoning, such as proteinuria and microscopic hematuria.
Treatment-induced nephrotoxicity is dose- dependent and may be reduced by assuring adequate diuresis before therapy begins. Urine flow must be monitored throughout therapy which must be stopped if anuria or severe oliguria develop. The proximal tubule hydropic degeneration usually recovers upon cessation of therapy. Edetate calcium disodium must be used in reduced doses in patients with pre-existing mild renal disease. Patients should be monitored for cardiac rhythm irregularities and other ECG changes during intravenous therapy.
Information for patients: Patients should be instructed to immediately inform their physician if urine output stops for a period of 12 hours. Laboratory tests: Urinalysis and urine sediment, renal and hepatic function and serum electrolyte levels should be checked before each course of therapy and then be monitored daily during therapy in severe cases, and in less serious cases after the second and fifth day of therapy. Therapy must be discontinued at the first sign of renal toxicity. The presence of large renal epithelial cells or increasing number of red blood cells in urinary sediment or greater proteinuria call for immediate stopping of edetate calcium disodium administration. Alkaline phosphatase values are frequently depressed (possibly due to decreased serum zinc levels), but return to normal within 48 hours after cessation of therapy. Elevated erythrocyte protoporphyrin levels (> 35 mcg/dl of whole blood) indicate the need to perform a venous blood lead determination. If the whole blood lead concentration is between 25-55 mcg/dl a mobilization test can be considered. (See Diagnostic Test.) An elevation of urinary coproporphyrin (adults: > 250 mcg/day; pediatric patients under 80 lbs: > 75 mcg/day) and elevation of urinary delta aminolevulinic acid (ALA) (adults: > 4 mg/day; pediatric patients: > 3 mg/m2/day) are associated with blood lead levels > 40 mcg/dl. Urinary coproporphyrin may be falsely negative in terminal patients and in severely iron-depleted pediatric patients who are not regenerating heme. In growing pediatric patients long bone x-rays showing lead lines and abdominal x-rays showing radio- opaque material in the abdomen may be of help in estimating the level of exposure to lead. Carcinogenesis, Mutagenesis, Impairment of Fertility: Long term animal studies have not been conducted with edetate calcium disodium to evaluate its carcinogenic potential, mutagenic potential or its effect on fertility. Pregnancy: Category B: One reproduction study was performed in rats at doses up to 13 times the human dose and revealed no evidence of impaired fertility or harm to the fetus due to Calcium Disodium Versenate. Another reproduction study performed in rats at doses up to about 25 to 40 times the human dose revealed evidence of fetal malformations due to Calcium Disodium Versenate, which were prevented by simultaneous supplementation of dietary zinc. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. Labor and Delivery: Calcium Disodium Versenate has no recognized use during labor and delivery, and its effects during these processes are unknown. Nursing Mothers: It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Calcium Disodium Versenate is administered to a nursing woman. Pediatric Use: Since lead poisoning occurs in pediatric populations and adults but is frequently more severe in pediatric patients, Calcium Disodium Versenate is used in patients of all ages. The intramuscular route is preferred by some for young pediatric patients. In cases where the intravenous route is necessary, avoid rapid infusion. (See WARNINGS.) Urine flow must be monitored throughout therapy; Calcium Disodium Versenate therapy must be stopped if anuria or severe oliguria develops. (See General Precautions.) At no time should the recommended daily dosage be exceeded. (See DOSAGE AND ADMINISTRATION.) |
| Adverse Reactions | Risk of kidney disorders, - Exceptional reactions which may occur during the hours following the injection: fever with malaise, vomiting and headaches, mainly observed with too quick injections, and also decrease of the blood pressure, nasal congestion. | Body as a Whole: pain at intramuscular injection site, fever, chills, malaise, fatigue, myalgia, arthralgia.
Cardiovascular: hypotension, cardiac rhythm irregularities. Renal: acute necrosis of proximal tubules (which may result in fatal nephrosis), infrequent changes in distal tubules and glomeruli. Urinary: glycosuria, proteinuria, microscopic hematuria and large epithelial cells in urinary sediment. Nervous System: tremors, headache, numbness, tingling. Gastrointestinal: cheilosis, nausea, vomiting, anorexia, excessive thirst. Hepatic: mild increases in SGOT and SGPT are common, and return to normal within 48 hours after cessation of therapy. Immunogenic: histamine-like reactions (sneezing, nasal congestion, lacrimation), rash. Hematopoietic: transient bone marrow depression, anemia. Metabolic: zinc deficiency, hypercalcemia. |
| Drug Interactions | None included. | Drug Interactions: There is no known drug interference with standard clinical laboratory tests. Steroids enhance the renal toxicity of edetate calcium disodium in animals. Edetate calcium disodium interferes with the action of zinc insulin preparations by chelating the zinc. |
| Overdosage | If you use more CALCIUM EDETATE DE SODIUM SERB 50 mg / ml, solution for I.V. injection than you should: High doses of calcium edetate may lead to renal tubular necrosis (destruction of the small tubes of the kidney). Please inform your doctor in case of too high dose administered. | Symptoms: Inadvertent administration of 5 times the recommended dose, infused intravenously over a 24 hour period, to an asymptomatic 16 month old patient with a blood lead content of 56 mcg/dl did not cause any ill effects. Edetate calcium disodium can aggravate the symptoms of severe lead poisoning, therefore, most toxic effects (cerebral edema, renal tubular necrosis) appear to be associated with lead poisoning. Because of cerebral edema, a therapeutic dose may be lethal to an adult or a pediatric patient with lead encephalopathy.
Higher dosage of edetate calcium disodium may produce a more severe zinc deficiency.
Treatment: Cerebral edema should be treated with repeated doses of mannitol. Steroids enhance the renal toxicity of edetate calcium disodium in animals and, therefore, are no longer recommended. Zinc levels must be monitored. Good urinary output must be maintained because diuresis will enhance drug elimination. It is not known if edetate calcium disodium is dialyzable. |
| Carton Picture | 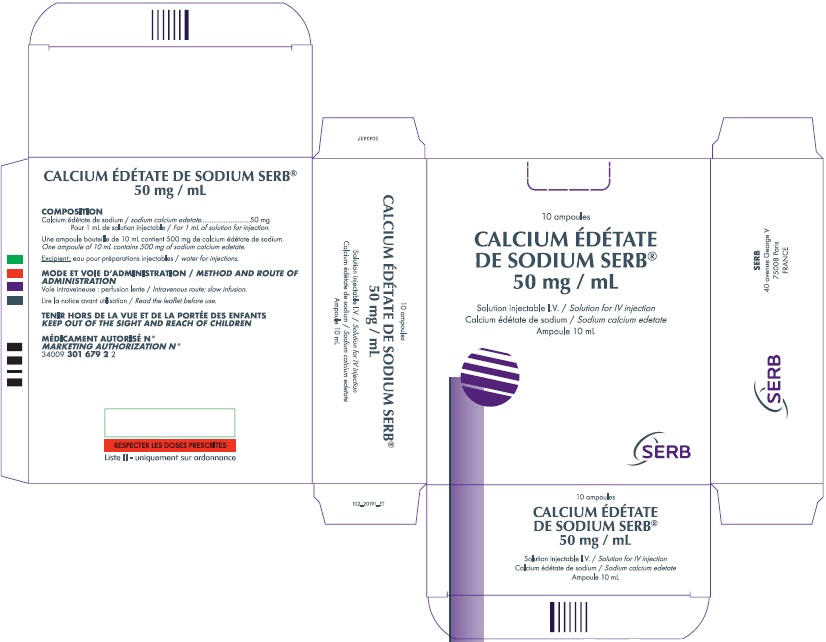 | 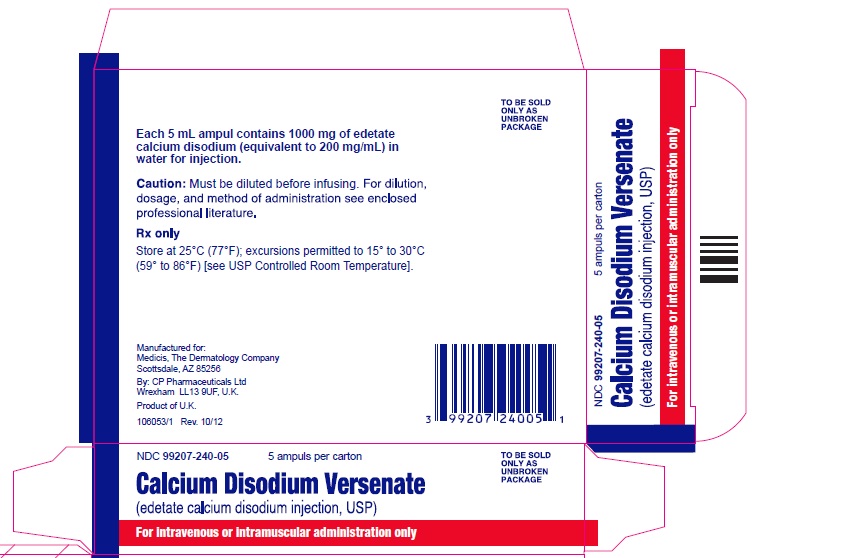 |
| CALCIUM EDETATE DE SODIUM
edetate calcium disodium anhydrous solution |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - BTG International Inc (617382395) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.