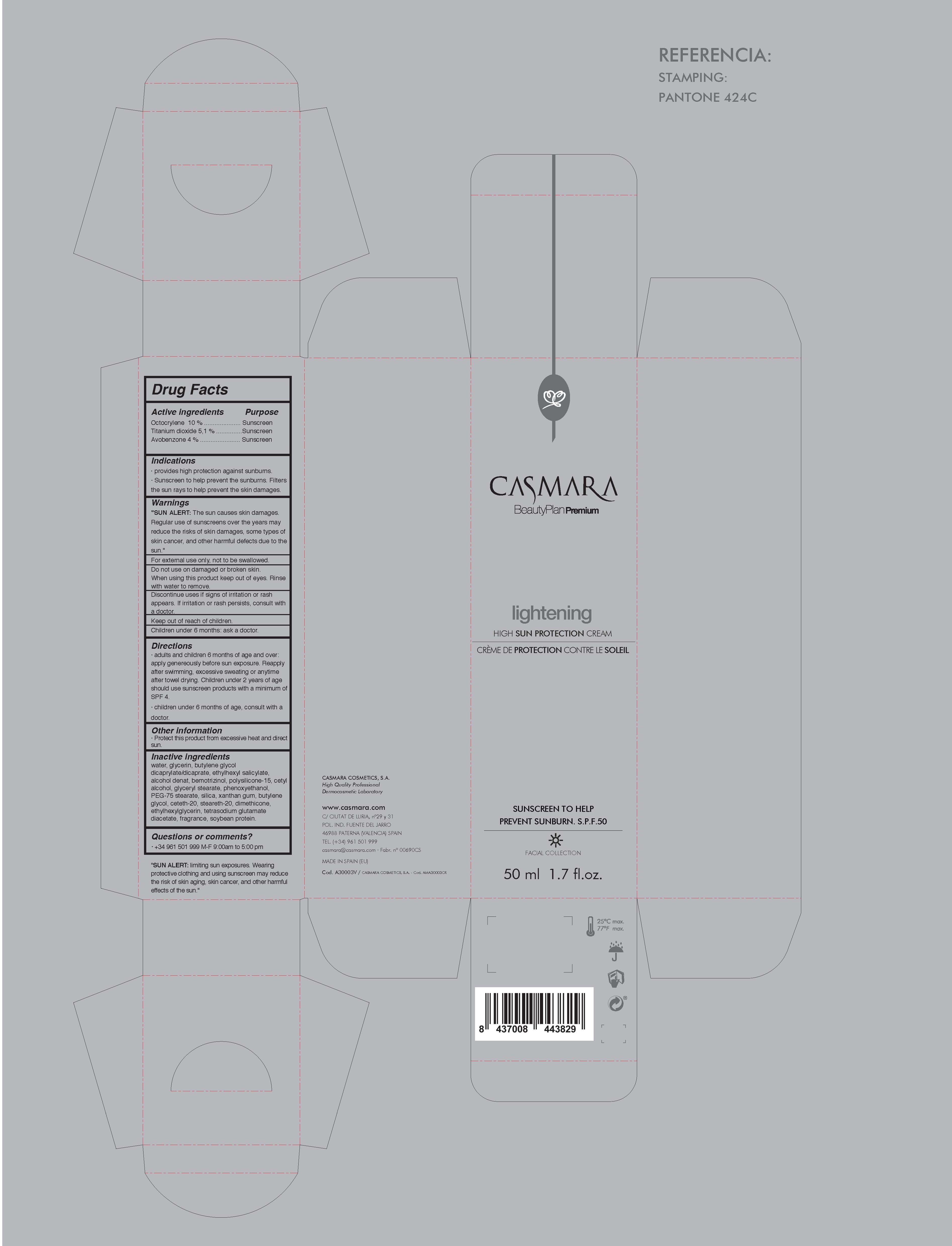
LIGHTENING HIGH SUN PROTECTION CREAM- octocrylene, titanium dioxide, avobenzone cream
Lightening High Sun Protection Cream by
Drug Labeling and Warnings
Lightening High Sun Protection Cream by is a Otc medication manufactured, distributed, or labeled by Casmara Cosmetics, SA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENTS PURPOSE
-
Warnings
SUN ALERT: The sun causes skin damages. Regular use of sunscreens over the years may reduce the risks of skin damages, some types of skin cancer, and other harmful effects due to the sun
For external use only, not to be swallowed.
Do not use on damaged or broken skin.
When using this product keep our of the eyes. Rinse with water to remove.
DIscontinue uses if signs or irritationsor rash appears. If irritation or rash persists, consult with a doctor.
Keep out of reach of children.
Children under 6 months: as a doctor.
- Questions or comments?
- Other Information
-
Directions
- adults and childrens 6 months of age and over: apply generaously before sun exposure. Reapply after swiming, excessive sweating or anytime after towel drying. Children under 2 years of age or less should use sunscreen products with a minium of SPF 4
- children under 6 months of age, consult with a doctor
- adults and childrens 6 months of age and over: apply generaously before sun exposure. Reapply after swiming, excessive sweating or anytime after towel drying. Children under 2 years of age or less should use sunscreen products with a minium of SPF 4
- children under 6 months of age, consult with a doctor
- Uses
-
Inactive Ingredients
Water, Glycerin, Butylene glycol dicaprylate/dicaprate, Ethylhexyl salicylate, Alcohol denat, Bemotrizinol, Polysilicone-15, Cetyl alcohol, Glyceryl stearate, Phenoxyethanol, PEG-75 stearate, Silica, Xanthan gum, Butylene glycol, Ceteth-20, Steareth-20, Dimethicone, Ethylhexylglycerin, Tetrasodium glutamate diacetate, Fragrance, Soybean protein
- Uses
- Package Label
-
INGREDIENTS AND APPEARANCE
LIGHTENING HIGH SUN PROTECTION CREAM
octocrylene, titanium dioxide, avobenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 20151-105 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 5.1 mg in 1 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 4 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) 0.12 mg in 1 mL SOYBEAN (UNII: L7HT8F1ZOD) 0.02 mg in 1 mL CETETH-20 (UNII: I835H2IHHX) 0.372 mg in 1 mL STEARETH-20 (UNII: L0Q8IK9E08) 0.372 mg in 1 mL BUTYLENE GLYCOL (UNII: 3XUS85K0RA) 0.5 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) 5 mg in 1 mL POLYSILICONE-15 (UNII: F8DRP5BB29) 3 mg in 1 mL ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) 0.1 mg in 1 mL WATER (UNII: 059QF0KO0R) 43.56 mg in 1 mL ALCOHOL (UNII: 3K9958V90M) 4.8 mg in 1 mL BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) 5 mg in 1 mL TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) 0.094 mg in 1 mL BEMOTRIZINOL (UNII: PWZ1720CBH) 4 mg in 1 mL GLYCERIN (UNII: PDC6A3C0OX) 7 mg in 1 mL CETYL ALCOHOL (UNII: 936JST6JCN) 1.87 mg in 1 mL GLYCERYL STEARATE SE (UNII: FCZ5MH785I) 1.875 mg in 1 mL PHENOXYETHANOL (UNII: HIE492ZZ3T) 0.9 mg in 1 mL PEG-75 STEARATE (UNII: OT38R0N74H) 0.872 mg in 1 mL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) 0.78 mg in 1 mL XANTHAN GUM (UNII: TTV12P4NEE) 0.6 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 20151-105-02 1 in 1 BOTTLE, DISPENSING 04/01/2017 1 NDC: 20151-105-01 50 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/29/2017 Labeler - Casmara Cosmetics, SA (464973544) Registrant - Casmara Cosmetics, SA (464973544) Establishment Name Address ID/FEI Business Operations Casmara Cosmetics, SA 464973544 manufacture(20151-105)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.