UNDA 19- aluminium metallicum, drosera, illicium verum, mentha piperita, senega officinalis liquid
UNDA 19 by
Drug Labeling and Warnings
UNDA 19 by is a Homeopathic medication manufactured, distributed, or labeled by Seroyal USA, SAN’UP. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
-
WARNINGS
Warnings
Ask a doctor before use if you have
Persistent or chronic cough such as
occurs with smoking, asthma, chronic
bronchitis, or emphysema.
Cough that is accompanied by
excessive phlegm (mucus) or fever.Stop use and ask a doctor if
Cough persists for more than 1 week,
tends to recur, or is accompanied by a
fever, rash, or persistent headache. A
persistent cough may be a sign of a
serious condition.
Symptoms persist or worsen.
If pregnant or breastfeeding, ask a
health professional before use.
Keep out of reach of children. In case
of overdose, get medical help or contact
a Poison Control Center right away. - KEEP OUT OF REACH OF CHILDREN
- PREGNANCY OR BREAST FEEDING
- ASK DOCTOR
- STOP USE
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
-
INDICATIONS & USAGE
Uses
For the temporary relief of symptoms associated with minor respiratory problems.Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or asrecommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
-
PRINCIPAL DISPLAY PANEL
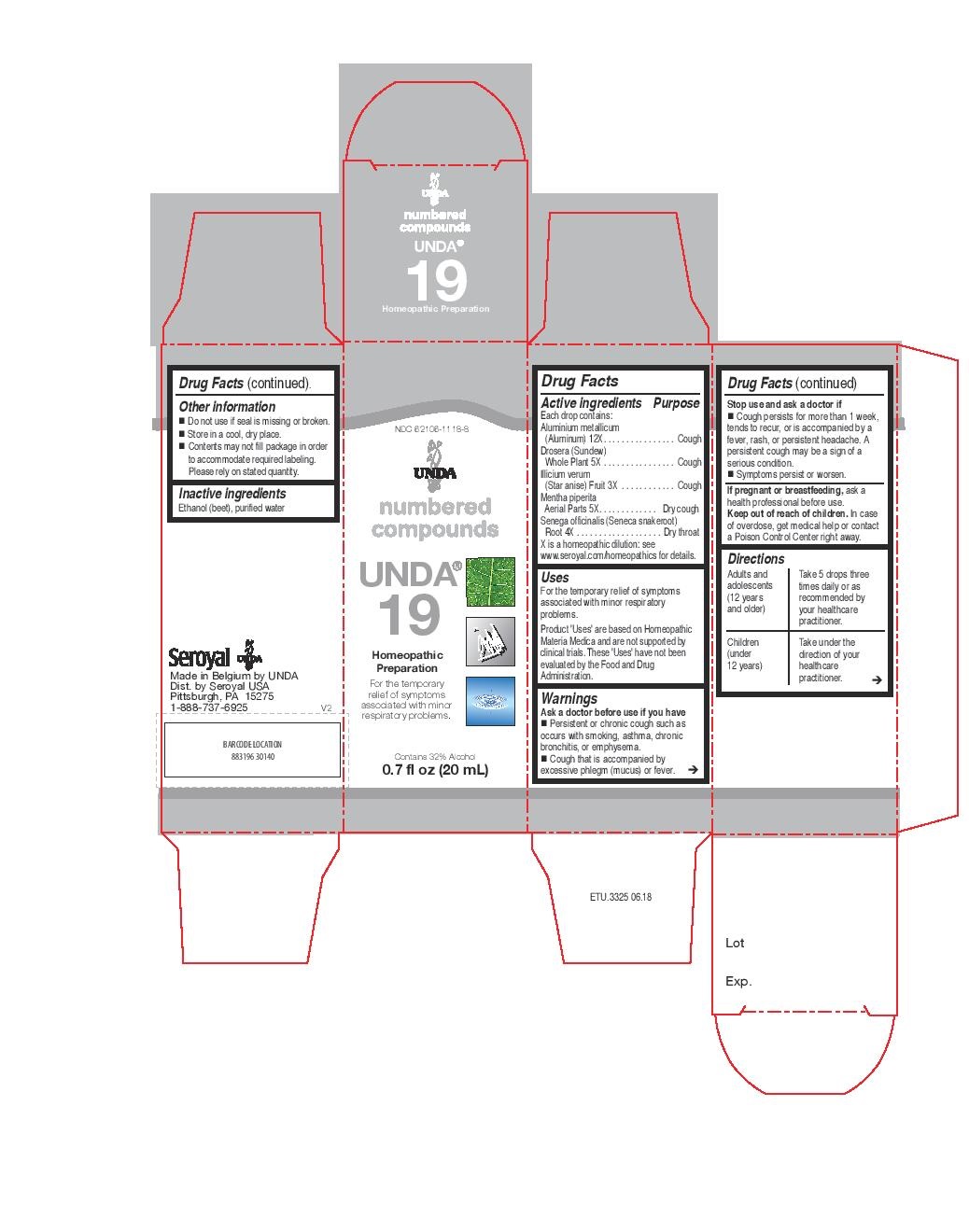
NDC: 62106-1118-8
UNDA
numbered compounds
UNDA 19
Homeopathic Preparation
For the temporary relief of symptoms
associated with minor respiratory problems.
Contains 32% Alcohol
0.7 fl oz (20 mL)
-
INGREDIENTS AND APPEARANCE
UNDA 19
aluminium metallicum, drosera, illicium verum, mentha piperita, senega officinalis liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 62106-1118 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM (UNII: CPD4NFA903) (ALUMINUM - UNII:CPD4NFA903) ALUMINUM 12 [hp_X] in 20 mL STAR ANISE FRUIT (UNII: CK15HA8438) (STAR ANISE FRUIT - UNII:CK15HA8438) STAR ANISE FRUIT 3 [hp_X] in 20 mL POLYGALA SENEGA ROOT (UNII: M7T6H7D4IF) (POLYGALA SENEGA ROOT - UNII:M7T6H7D4IF) POLYGALA SENEGA ROOT 4 [hp_X] in 20 mL DROSERA ROTUNDIFOLIA FLOWERING TOP (UNII: 75O014T1HG) (DROSERA ROTUNDIFOLIA FLOWERING TOP - UNII:75O014T1HG) DROSERA ROTUNDIFOLIA FLOWERING TOP 5 [hp_X] in 20 mL PEPPERMINT (UNII: V95R5KMY2B) (PEPPERMINT - UNII:V95R5KMY2B) PEPPERMINT 5 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62106-1118-8 1 in 1 CARTON 04/03/2017 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 04/03/2017 Labeler - Seroyal USA (018361118) Establishment Name Address ID/FEI Business Operations SAN’UP 401010287 manufacture(62106-1118)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.