DOJOLVI- triheptanoin liquid
DOJOLVI by
Drug Labeling and Warnings
DOJOLVI by is a Prescription medication manufactured, distributed, or labeled by Ultragenyx Pharmaceutical Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use DOJOLVI safely and effectively. See full prescribing information for DOJOLVI.
DOJOLVI® (triheptanoin) oral liquid
Initial U.S. Approval: 2020RECENT MAJOR CHANGES
Dosage and Administration (2.4) 10/2023 INDICATIONS AND USAGE
DOJOLVI is a medium-chain triglyceride indicated as a source of calories and fatty acids for the treatment of adult and pediatric patients with molecularly confirmed long-chain fatty acid oxidation disorders (LC-FAOD). (1)
DOSAGE AND ADMINISTRATION
- Assess metabolic requirements by determining daily caloric intake (DCI) prior to calculating the dose of DOJOLVI. (2.1)
- For patients receiving another medium-chain triglyceride product, discontinue prior to the first dose of DOJOLVI. (2.3)
- The recommended target daily dosage of DOJOLVI is up to 35% of the patient's total prescribed DCI divided into at least four doses and mixed thoroughly into semi-solid food/liquid or medical food/formula at mealtimes or with snacks. (2.2)
- See the full prescribing information for instructions on how to calculate the volume per dose; initiate and titrate the dosage to achieve the target; and prepare and administer DOJOLVI. (2.2, 2.3, 2.4)
DOSAGE FORMS AND STRENGTHS
Oral Liquid, 100% w/w of triheptanoin. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Feeding Tube Dysfunction: Regularly monitor the feeding tube to ensure proper functioning and integrity. (5.1)
- Intestinal Malabsorption in Patients with Pancreatic Insufficiency: Low or absent pancreatic enzymes may reduce absorption of DOJOLVI. Avoid administration of DOJOLVI in patients with pancreatic insufficiency. (5.2)
ADVERSE REACTIONS
Most common adverse reactions are (≥10%): abdominal pain, diarrhea, vomiting, and nausea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Ultragenyx Pharmaceutical Inc. at 1-888-756-8657 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Pancreatic Lipase Inhibitors: Avoid co-administration due to potential for reduced clinical effect of DOJOLVI. (7.1)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 10/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Recommendations Prior to DOJOLVI Treatment
2.2 Recommended Dosage
2.3 Dosage Initiation and Titration
2.4 Preparation and Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Feeding Tube Dysfunction
5.2 Intestinal Malabsorption in Patients with Pancreatic Insufficiency
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
7 DRUG INTERACTIONS
7.1 Pancreatic Lipase Inhibitors
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Recommendations Prior to DOJOLVI Treatment
All patients treated with DOJOLVI should be under the care of a clinical specialist knowledgeable in appropriate disease-related dietary management based upon current nutritional recommendations.
Assess the metabolic requirements of the patient by determining their daily caloric intake (DCI) prior to calculating the dose of DOJOLVI.
For patients receiving another medium-chain triglyceride (MCT) product, discontinue prior to the first dose of DOJOLVI.
2.2 Recommended Dosage
The recommended target daily dosage of DOJOLVI is up to 35% of the patient's total prescribed DCI divided into at least four doses and administered by mixing thoroughly into semi-solid food/liquid or medical food/formula at mealtimes or with snacks.
In order to reach a target daily dosage, patients may require an increase in their total fat intake.
The neonatal population may require higher fat intake and therefore an increased amount of DOJOLVI. Consider current nutritional recommendations when dosing the neonatal population.
Total Daily Dosage Calculation
The target daily dosage from DOJOLVI (%) is converted to a volume of DOJOLVI (mL) to be administered using the following calculation:
- Caloric value of DOJOLVI = 8.3 kcal/mL
- Round the total daily dosage to the nearest whole milliliter.
- Divide the total daily dosage into at least four approximately equal individual doses.
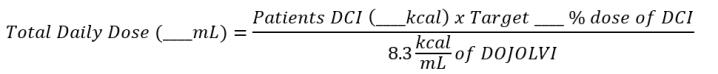
2.3 Dosage Initiation and Titration
For patients not currently taking an MCT product
Initiate DOJOLVI at a total daily dosage of approximately 10% DCI divided into at least four times per day. Increase to the recommended total daily dosage by approximately 5% DCI every 2 to 3 days until the target dosage of up to 35% DCI is achieved.
For patients switching from another MCT product
Discontinue use of MCT products before starting DOJOLVI.
Initiate DOJOLVI at the last tolerated daily dosage (mL) of MCT divided into at least four times per day. Increase the total daily dosage by approximately 5% DCI every 2 to 3 days until the target dosage of up to 35% DCI is achieved.
Tolerability
- Consider more frequent smaller doses if a patient has difficulty tolerating 1/4 of the total daily dosage at one time based on gastrointestinal adverse reactions [see Adverse Reactions (6.1)].
- Monitor the patient's total caloric intake during dosage titration, especially in a patient with gastrointestinal adverse reactions, and adjust all components of the diet as needed.
- If a patient experiences gastrointestinal adverse reaction(s), consider dosage reduction until the gastrointestinal symptoms resolve [see Adverse Reactions (6.1)].
- If a patient is unable to achieve the target daily dosage of up to 35% DCI during dosage titration, maintain the patient at the maximum tolerated dosage.
2.4 Preparation and Administration Instructions
Administer DOJOLVI by mixing thoroughly with semi-solid food/liquid (oral administration) or medical food/formula (feeding tube administration). Do not administer DOJOLVI alone to avoid gastrointestinal upset and feeding tube degradation [see Adverse Reactions (6.1)].
Prepare or administer DOJOLVI using containers, oral syringes, or measuring cups made of compatible materials such as stainless steel, glass, high density polyethylene (HDPE), polypropylene, low density polyethylene, polyurethane, and silicone.
Do not prepare or administer DOJOLVI using containers, oral syringes, or measuring cups made of polystyrene or polyvinyl chloride (PVC) plastics.
Regularly monitor the containers, dosing components, or utensils that are in contact with DOJOLVI to ensure proper functioning and integrity.
Oral Preparation and Administration
- Use an oral syringe or measuring cup made of compatible materials as listed above to withdraw the prescribed volume of DOJOLVI from the bottle.
- DOJOLVI can be mixed with soft food or liquid such as:
- plain or artificially sweetened fat free yogurt
- fat free milk, formula, or cottage cheese
- whole grain hot cereal
- fat free low carbohydrate pudding, smoothies, applesauce, or baby food
- Add the prescribed amount of DOJOLVI to a clean bowl, cup, or container, made of the compatible materials as listed above, which contains an appropriate amount of semi-solid food or liquid that takes into consideration the age, size, and fluid needs of the patient to ensure administration of the full dose.
- Mix DOJOLVI thoroughly into the food or liquid.
- Any unused mixture may be stored for up to 24 hours in refrigerated conditions.
- If not used within 24 hours, discard DOJOLVI mixture in the trash. Do not pour down the sink. Do not save for later.
Feeding Tube Preparation and Administration
DOJOLVI is administered as an oral or enteral bolus medication. Do not add DOJOLVI to the feeding bag, as the feeding equipment may degrade over time.
DOJOLVI can be administered via oral or enteral feeding tubes manufactured of silicone or polyurethane. Do not use feeding tubes manufactured of polyvinyl chloride (PVC). Feeding device performance and functionality can degrade over time depending on usage and environmental conditions. Regularly monitor the feeding tube to ensure proper functioning and integrity [see Warnings and Precautions (5.1)].
- Use an oral syringe or measuring cup made of compatible materials as listed above to withdraw the prescribed volume of DOJOLVI from the bottle.
- Add the prescribed amount of DOJOLVI to a clean bowl, cup, or container, made of compatible materials as listed above, which contains an amount of medical food or formula that takes into consideration the age, size, and fluid needs of the patient in order to ensure administration of the full dose.
- Mix DOJOLVI thoroughly into the medical food or formula prior to administering via feeding tube, y-connector, or feeding tube extension set made of silicone or polyurethane.
- Draw up the entire amount of the DOJOLVI mixture into a slip tip syringe.
- Remove the residual air from the syringe and connect the syringe directly into the feeding tube port.
- Push the syringe contents into the feeding tube port using steady pressure until empty.
- Flush the feeding tubes with between 5 mL to 30 mL of water. Flush volume should be modified based on specific patient needs and in cases of fluid restriction.
- Discard any unused DOJOLVI mixture in the trash. Do not pour down the sink. Do not save for later use.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Feeding Tube Dysfunction
Feeding tube performance and functionality can degrade over time depending on usage and environmental conditions. In clinical trials, feeding tube dysfunction was reported in patients receiving triheptanoin. The contribution of DOJOLVI cannot be ruled out. Do not administer DOJOLVI in feeding tubes manufactured of polyvinyl chloride (PVC) [see Dosage and Administration (2.4]. Regularly monitor the feeding tube to ensure proper functioning and integrity.
5.2 Intestinal Malabsorption in Patients with Pancreatic Insufficiency
Pancreatic enzymes hydrolyze triheptanoin and release heptanoate as medium-chain fatty acids in the small intestine. Low or absent pancreatic enzymes may result in reduced absorption of heptanoate subsequently leading to insufficient supplementation of medium-chain fatty acids. Avoid administration of DOJOLVI in patients with pancreatic insufficiency.
-
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety population included 79 patients with LC-FAOD exposed to DOJOLVI in two studies: one open-label 78-week study of DOJOLVI in 29 patients (Study 1) followed by an open-label extension study (Study 2). Twenty-four patients from Study 1 continued into Study 2. Patients ranged from 4 months to 63 years of age and the population was 52% male. Of the 79 patients, 87% were White, 5% were Black or African-American, 4% were Asian, and 4% other. The daily dosage of DOJOLVI ranged between 12% and 41% DCI (which corresponds to 0.7 g/kg/day to 6.0 g/kg/day for pediatric patients and 0.5 g/kg/day to 1.3 g/kg/day for adult patients) for a mean duration of 23 months.
The most common adverse reactions to DOJOLVI reported in the pooled safety population of Study 1 and Study 2 were gastrointestinal (GI)-related, and included abdominal pain (abdominal discomfort, abdominal distension, abdominal pain, abdominal pain upper, GI pain) [60%], diarrhea [44%], vomiting [44%], and nausea [14%].
Gastrointestinal (GI) Adverse Reactions
In Study 1 and Study 2, median time to onset of a first occurrence of a GI adverse reaction was 7.3 weeks. GI adverse reactions led to dose reductions in 35% and 12% of patients in Study 1 and Study 2, respectively.
In Study 3, a 4-month double-blind randomized controlled study, commonly reported adverse reactions with triheptanoin were similar to those reported in Study 1 and Study 2.
-
7 DRUG INTERACTIONS
7.1 Pancreatic Lipase Inhibitors
Co-administration of triheptanoin with a pancreatic lipase inhibitor (e.g., orlistat) may reduce exposure to the triheptanoin metabolite, heptanoate, and reduce the clinical effect of triheptanoin [see Clinical Pharmacology (12.3)]. Avoid co-administration of DOJOLVI with pancreatic lipase inhibitors.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on triheptanoin use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. In animal reproduction studies conducted in pregnant rats and rabbits administered triheptanoin during the period of organogenesis, the primary toxicological effect (reduced body weight gain) was considered to be specific to decreased food consumption related to taste aversion in animals, and therefore is not relevant to clinical use in the intended populations.
There is a pregnancy safety study for DOJOLVI. If a patient becomes pregnant while receiving DOJOLVI, healthcare providers should report DOJOLVI exposure by calling Ultragenyx Pharmaceutical Inc. at 1-888-756-8657.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Embryofetal developmental studies have been conducted with triheptanoin in rats and rabbits following oral administration of 10% (3.2 g/kg), 30% (9.7 g/kg) and 50% (16 g/kg) DCI in rats and 10% (1.2 g/kg), 20% (2.3 g/kg) and 30% (3.5 g/kg) DCI in rabbits during the period of organogenesis. Reduced body weight gain, associated with decreased food consumption, was observed in pregnant rats and rabbits following administration of triheptanoin food mixture and was attributed to taste aversion. The NOAEL for this maternal toxicity (lack of body weight gain) was 10% DCI for both rats and rabbits. Administration of dietary triheptanoin to pregnant rats at doses approximately 2 times above, and pregnant rabbits approximately equal to the targeted clinical dose of 35% DCI resulted in increased incidence of skeletal malformations and decreased litter weights in both species and reduced number of viable litters in rabbits. The adverse effects on rat and rabbit embryofetal development were associated with the reduced body weight gain observed in pregnant animals. The NOAEL for embryofetal development toxicity was 30% and 20% DCI for rats and rabbits, respectively. In a pre- and postnatal developmental study in rats, reduced birthweights and delayed sexual maturation in pups were observed at 50% DCI and were considered secondary to the reductions in body weight gain in pregnant rats.
8.2 Lactation
Risk Summary
There are no data on the presence of triheptanoin or its metabolites in human or animal milk, the effects on the breastfed infant, or the effects on milk production. Medium-chain triglycerides and other fatty acids are normal components of breastmilk and the composition of breastmilk varies within feedings, over stages of lactation, and between mothers and populations due to maternal factors including genetics, environment, and diet. The developmental and health benefits of breastfeeding should be considered along with the clinical need for DOJOLVI and any potential adverse effect on the breastfed infant from DOJOLVI or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of DOJOLVI have been established in pediatric patients [see Adverse Reactions (6.1), Clinical Studies (14)].
-
11 DESCRIPTION
DOJOLVI (triheptanoin) is a synthetic medium odd-chain (C7) triglyceride supplied as a colorless to light yellow clear oral liquid. The chemical name of triheptanoin is heptanoic acid, 1,1',1''-(1,2,3-propanetriyl) ester. The empirical formula is C24H44O6 and its molecular weight is 428.6 g/mol. The chemical structure is:
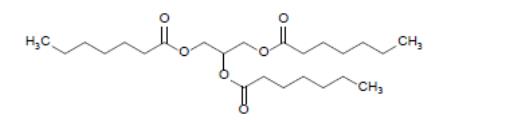
The caloric value of triheptanoin is 8.3 kcal/mL. The fat content is 0.96 g/mL.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Triheptanoin is a medium-chain triglyceride consisting of three odd-chain 7-carbon length fatty acids (heptanoate) that provide a source of calories and fatty acids to bypass the long-chain FAOD enzyme deficiencies for energy production and replacement.
12.3 Pharmacokinetics
Following oral administration, triheptanoin is extensively hydrolyzed to heptanoate and glycerol by pancreatic lipases in the intestines. The exposure of triheptanoin in the human plasma is minimal. Pharmacokinetics of heptanoate exhibits high inter-patient variability. Heptanoate exposure increases greater than dose-proportional in the dose range between triheptanoin 0.3 and 0.4 g/kg.
Absorption
The pharmacokinetics of heptanoate in healthy adult subjects following an oral administration of DOJOLVI mixed with food are summarized in Table 1.
Table 1: Summary of Pharmacokinetic Parameters of Heptanoate after Single and Multiple Oral Administration of DOJOLVI to Healthy Adults (N = 13) DOJOLVI Dose Mean (SD)
Cmax
(µmol/L)Mean (SD)
AUC0-8h
(µmol*hr/L)Time to First Peak Concentration*
Median (range)
(hours)- * After oral administration of DOJOLVI, more than one peak concentration of heptanoate is observed.
Single
Dose0.3 g/kg 178.9 (145) 336.5 (223) 0.5 (0.4 to 1.0) 0.4 g/kg 259.1 (134) 569.1 (189) 0.8 (0.4 to 6.4) Multiple
Doses0.3 g/kg administered 4 times a day for 2 days
(total daily dosage of 1.3 g/kg/day)319.9 (164) 789.8 (346) 1.2 (0.0 to 2.4) Distribution
The plasma protein binding of heptanoate is approximately 80% and is independent of total concentration.
Elimination
After a single dose of either 0.3 g/kg or 0.4 g/kg triheptanoin to healthy subjects, the mean apparent clearance (CL/F) of heptanoate was 6.05 and 4.31 L/hr/kg, respectively. Half-life (t1/2) of heptanoate could not be determined due to multiple peak concentrations of heptanoate observed.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Nonclinical animal studies evaluating long-term administration of triheptanoin have not been conducted to assess the carcinogenic potential of the drug. In a published chronic 9-month dietary study conducted in rats, daily administration of triheptanoin at dose levels up to 1.14 g/kg was associated with atrophy or hyperplasia of the intestinal villa. In a chronic 9-month dietary study conducted in juvenile minipigs, treatment with triheptanoin at dose levels up to 10 g/kg was well tolerated with no changes in histopathology suggestive of any carcinogenic potential.
Published studies with structurally similar triglycerides (i.e., MCTs) were also evaluated. In a 2-year dietary study of rats fed tricaprylin (C8 MCT) at dose levels up to 9.5 g/kg (approximately 1.2 times the anticipated maximum clinical dose), there were increased incidences of pancreatic and forestomach hyperplasia and adenomas but not carcinomas. Chronic administration of a diet containing approximately 17% MCT was not shown to promote effects on colon tumor incidence in an azomethane-induced colon tumorigenicity rat model.
Mutagenesis
Triheptanoin was not genotoxic in a battery of genotoxicity tests including the in vitro bacterial reverse mutation in S. typhimurium and E. coli, in vitro mammalian chromosomal aberration test in human peripheral blood lymphocytes and the in vivo mammalian erythrocyte micronucleus test in rat bone marrow.
Impairment of Fertility
Triheptanoin had no effect on fertility or any other parameters of mating performance in rats exposed to repeat dietary administration at dose levels equivalent to up to 50% daily caloric intake (16 g/kg) that resulted in systemic drug exposure (AUC) of heptanoate approximately equal to the maximum recommended human dose.
-
14 CLINICAL STUDIES
The efficacy of triheptanoin as a source of calories and fatty acids was evaluated in Study 3 (NCT01379625), a 4-month double-blind randomized controlled study comparing triheptanoin (7-carbon chain fatty acid) with trioctanoin (8-carbon chain fatty acid). The study enrolled 32 adult and pediatric patients with a confirmed diagnosis of LC-FAOD and evidence of at least one significant episode of rhabdomyolysis and at least two of the following diagnostic criteria: disease specific elevation of acylcarnitines on a newborn blood spot or in plasma, low enzyme activity in cultured fibroblasts, or one or more known pathogenic mutations in CPT2, ACADVL, HADHA, or HADHB.
The dosage of study drug was titrated to a protocol-specified target of 20% DCI (actual mean daily dose achieved was 16% for triheptanoin and 14% for trioctanoin). The recommended target dosage of DOJOLVI is up to 35% of DCI [see Dosage and Administration (2.2)]. Patients ranged in age from 7 years to 64 years (median 24 years) and 12 were male. Of the 32 patients, 100% were White and 3% were Hispanic.
Baseline cardiovascular function in both groups was normal and within test/retest variability normally observed in repeated echocardiograms. After 4 months, patients in both groups had similar mean changes from baseline in left ventricular ejection fraction and wall mass on resting echocardiogram and similar maximal heart rates on treadmill ergometry.
Five patients experienced 7 events of rhabdomyolysis in the triheptanoin group and 4 patients experienced 7 events of rhabdomyolysis in the trioctanoin group.
No differences were observed between triheptanoin and trioctanoin groups in blood markers of metabolism including glucose, insulin, lactate, total serum, ketones, acylcarnitines, and serum-free fatty acid concentrations.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
DOJOLVI (triheptanoin) oral liquid is supplied in a glass bottle as follows:
500 mL bottle NDC: 69794-050-50 Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) (see USP Controlled Room Temperature). Do not freeze.
DOJOLVI can be used for up to 9 months after opening but not beyond the expiration date on the bottle.
Do not dose or store using materials made of polystyrene or polyvinyl chloride (PVC) containers [see Dosage and Administration (2.4)].
Pharmacist: Dispense only in glass or HDPE bottles.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient and/or caregiver to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Preparation and Administration
Instruct the patient or caregiver:
- To read the Instructions for Use for appropriate preparation and administration instructions on oral versus feeding tube administration.
- Before administration, to mix DOJOLVI thoroughly into semi-solid food/liquid (oral) or medical food/formula (feeding tube) at mealtimes or with snacks.
- To not prepare or administer DOJOLVI using containers, oral syringes, or measuring cups made of polystyrene or polyvinyl chloride (PVC) plastics.
- That if a dose is missed, to take the next dose as soon as possible with subsequent doses taken at 3 to 4-hour intervals. Skip the missed dose if it will not be possible to take all doses in a day [see Dosage and Administration (2.2)].
Storage
Instruct the patient or caregiver to store DOJOLVI at room temperature in the bottle in which it was dispensed [see How Supplied/Storage and Handling (16)].
Feeding Tube Dysfunction
Advise the patient or caregiver to regularly monitor the feeding tube for proper functioning and integrity and report to the healthcare provider if any issues are identified [see Warnings and Precautions (5.1)].
Intestinal Malabsorption in Patients with Pancreatic Insufficiency
Inform the patient or caregiver that pancreatic insufficiency may reduce the clinical effect of DOJOLVI. Any known pancreatic insufficiency should be reported to the healthcare provider [see Warnings and Precautions (5.2)].
Pregnancy
Advise a patient who is exposed to DOJOLVI during pregnancy that there is a pregnancy safety study that monitors pregnancy outcomes. Encourage the patient to report the pregnancy to Ultragenyx Pharmaceutical Inc. at 1-888-756-8657 [see Use in Specific Populations (8.1)].
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 10/2023 PATIENT INFORMATION
DOJOLVI (doh-johl-vee)
(triheptanoin)
oral liquidWhat is DOJOLVI?
DOJOLVI is a prescription medicine used to treat long-chain fatty acid oxidation disorders (LC-FAOD) in children and adults.Before taking DOJOLVI, tell your healthcare provider about all of your medical conditions, including if you: - are pregnant or plan to become pregnant. It is not known if DOJOLVI will harm your unborn baby.
Pregnancy Safety Study. There is a pregnancy safety study for women who take DOJOLVI during pregnancy. The purpose of this study is to collect information about your health and your baby's health. You can talk to your healthcare provider or contact 1-888-756-8657 to enroll in this study or get more information. - are breastfeeding or plan to breastfeed. It is not known if DOJOLVI passes into breast milk. Talk to your healthcare provider about the best way to feed your baby if you take DOJOLVI.
- are taking a pancreatic lipase inhibitor, such as orlistat, as it may affect how well DOJOLVI works.
How should I take DOJOLVI? - See the detailed "Instructions for Use" at the end of this Patient Information Leaflet for instructions about how to mix and take DOJOLVI by mouth in soft food or drink or how to mix and give DOJOLVI in medical food or formula through feeding tubes.
- Take DOJOLVI exactly as your healthcare provider tells you.
- Your healthcare provider may start you on a low dose of DOJOLVI and slowly increase your dose to help avoid side effects. If you are taking another medium chain triglyceride (MCT) product, stop taking the MCT before starting DOJOLVI.
- Do not mix or give DOJOLVI using containers, oral syringes, or measuring cups made of polystyrene (a type of plastic that can be solid or foam) or polyvinyl chloride (PVC), a solid plastic material.
- Take DOJOLVI at least 4 times a day with meals or snacks, and always mix well with soft food or drink or medical food or formula before taking.
What are the possible side effects of DOJOLVI? - Feeding tube problems. Feeding tubes may not work as well or stop working over time when taking DOJOLVI. Do not use DOJOLVI in feeding tubes made of polyvinyl chloride (PVC). Check the feeding tube to make sure it is working properly and not breaking down.
- Intestinal absorption problems in patients with pancreatic insufficiency. If you have pancreatic insufficiency, consult with your healthcare provider as it may affect how well DOJOLVI works.
- The most common side effects of DOJOLVI include:
- stomach (abdominal) pain
- diarrhea
- vomiting
- nausea
These are not all the possible side effects of DOJOLVI. Call your healthcare provider for medical advice about side effects. You may report side effects to Ultragenyx at 1-888-756-8657 or FDA at 1-800-FDA-1088. How should I store DOJOLVI? - Store DOJOLVI at room temperature between 68°F to 77°F.
- Do not freeze DOJOLVI.
- When the bottle of DOJOLVI has been opened, use within 9 months or by the expiration date on the bottle, whichever comes first.
- Do not store DOJOLVI in containers made of polystyrene or polyvinyl chloride (PVC).
General information about the safe and effective use of DOJOLVI.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet. Do not use DOJOLVI for a condition for which it was not prescribed. Do not give DOJOLVI to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about DOJOLVI that is written for health professionals.What are the ingredients in DOJOLVI?
DOJOLVI is made of 100% triheptanoin and contains no other ingredients.
Manufactured for:
Ultragenyx Pharmaceutical Inc.
60 Leveroni Court
Novato, CA 94949
For more information, go to www.dojolvi.com or call 1-888-756-8657. - are pregnant or plan to become pregnant. It is not known if DOJOLVI will harm your unborn baby.
-
INSTRUCTIONS FOR USEDOJOLVI (doh-johl-vee)(triheptanoin)oral liquid
This Instructions for Use contains information on how to take DOJOLVI. Read this Instructions for Use before you start taking DOJOLVI and each time you get a refill. There may be new information. This Instructions for Use does not take the place of talking to your healthcare provider about your medical condition or treatment.
Important Information You Need to Know Before Taking or Giving DOJOLVI:
- Use an oral syringe or measuring cup to measure your prescribed dose. Ask your healthcare provider or pharmacist to show you how to measure your prescribed dose.
- Mix or give DOJOLVI using containers, oral syringes, or measuring cups made of materials such as stainless steel, glass, or high density polyethylene (HDPE), polypropylene, low density polyethylene, polyurethane, and silicone (types of plastic materials).
- After use, rinse materials and containers well with cold water and wipe dry.
- Do not mix or give DOJOLVI using containers, oral syringes, or measuring cups made of polystyrene (a type of plastic that can be solid or foam) or polyvinyl chloride (PVC), a solid plastic material.
- Take DOJOLVI at least 4 times a day with meals or snacks, and always mix well with soft food or drink or medical food or formula before taking.
- If you take DOJOLVI by mouth, mix well with soft food or drink such as:
- plain or artificially sweetened fat free yogurt
- fat free milk, formula, or cottage cheese
- whole grain hot cereal
- fat free low carbohydrate pudding, smoothies, applesauce, or baby food
- If you give DOJOLVI by feeding tube, mix well with medical food or formula.
- Your healthcare provider should advise you on how to maintain a proper diet when taking DOJOLVI.
- If you miss a dose, take the next dose as soon as possible. Take the following doses 3 to 4 hours apart. If it is not possible to take all the doses for the day, skip the missed dose.
Taking DOJOLVI by mouth:
- Use an oral syringe or measuring cup made of the materials listed above to measure the prescribed amount of DOJOLVI from the bottle.
- Add the prescribed amount of DOJOLVI to a clean bowl, cup, or container, made of the materials listed above, containing the appropriate amount of soft food or drink as instructed by your healthcare provider.
- Mix DOJOLVI well into the soft food or liquid and swallow the mixture.
- Do not take DOJOLVI alone to avoid stomach upset.
- You can store any unused DOJOLVI mixture for up to 24 hours in the refrigerator.
- If not used within 24 hours, throw away (dispose of) the DOJOLVI mixture in the trash. Do not pour down the sink. Do not save for later.
- Check the items used to take DOJOLVI often to make sure they are working properly and are not breaking down.
Giving DOJOLVI by feeding tube:
- You can give DOJOLVI by a feeding tube.
- Mix DOJOLVI with medical food or formula before giving by a feeding tube, y-connector, or feeding tube extension set.
- Only give DOJOLVI in a feeding tube made of silicone or polyurethane.
- Do not give DOJOLVI in a feeding tube made of polyvinyl chloride (PVC), a type of plastic.
- Do not give DOJOLVI alone to avoid stomach upset and to avoid breakdown of the feeding tube.
- Do not add DOJOLVI to the feeding bag because the feeding equipment may break down over time.
- Use an oral syringe or measuring cup made of the materials listed above to measure the prescribed amount of DOJOLVI from the bottle.
- Add the prescribed amount of DOJOLVI to a clean bowl, cup, or container, made of the materials listed above, containing the appropriate amount of medical food or formula as instructed by your healthcare provider.
- Mix DOJOLVI well into the medical food or formula and draw up the entire amount of the mixture into a slip tip syringe.
- Remove the air from the syringe and connect the syringe directly into the feeding tube port.
- Push the contents of the syringe (DOJOLVI mixture) into the feeding tube port using steady pressure until empty.
- Draw up about 5 mL to 30 mL of water with the slip tip syringe and flush the feeding tube feeding port with the water.
- Throw away (dispose of) any unused DOJOLVI mixture in the trash. Do not pour down the sink. Do not save for later use.
- Check the feeding tube and other items used to give DOJOLVI often to make sure they are working properly and are not breaking down.
How should I store DOJOLVI?
- Store DOJOLVI at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not freeze DOJOLVI.
- When the bottle of DOJOLVI has been opened, use within 9 months or by the expiration date on the bottle, whichever comes first.
- Do not store DOJOLVI in containers made of polystyrene or polyvinyl chloride (PVC).
Keep DOJOLVI and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.Revised: 10/2023
-
PRINCIPAL DISPLAY PANEL - 500 mL Bottle Label
NDC: 69794-050-50
DOJOLVI®
TRIHEPTANOIN
Oral liquid, 100% w/w
Rx onlyAttention: For use with compatible materials
only. See Prescribing Information. Do not use
with polystyrene or polyvinylchloride plastics.ultragenyx
500 mL
-
PRINCIPAL DISPLAY PANEL - 500 mL Bottle Carton
NDC: 69794-050-50
DOJOLVI®
TRIHEPTANOIN
Oral liquid, 100% w/w
Rx onlyAttention: For use with compatible materials
only. See Prescribing Information. Do not use
with polystyrene or polyvinylchloride plastics.500 mL
1 bottleultragenyx

-
INGREDIENTS AND APPEARANCE
DOJOLVI
triheptanoin liquidProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 69794-050 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRIHEPTANOIN (UNII: 2P6O7CFW5K) (TRIHEPTANOIN - UNII:2P6O7CFW5K) TRIHEPTANOIN 0.96 g in 1 mL Product Characteristics Color YELLOW (colorless to light yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69794-050-50 1 in 1 CARTON 07/01/2020 1 500 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213687 07/01/2020 Labeler - Ultragenyx Pharmaceutical Inc. (962892019) Registrant - Ultragenyx Pharmaceutical Inc. (962892019) Establishment Name Address ID/FEI Business Operations Ultragenyx Pharmaceutical Inc. 962892019 ANALYSIS(69794-050) Establishment Name Address ID/FEI Business Operations AndersonBrecon Inc. (PCI Pharma Services) USA 053217022 PACK(69794-050) Establishment Name Address ID/FEI Business Operations GBA Pharma GMbH 342374604 ANALYSIS(69794-050) Establishment Name Address ID/FEI Business Operations Labor LS SE & Co. KG 314929072 ANALYSIS(69794-050) Establishment Name Address ID/FEI Business Operations Integrated Commercialization Solutions, LLC 832820588 PACK(69794-050) Establishment Name Address ID/FEI Business Operations Haupt Pharma Amareg GmbH 331334909 MANUFACTURE(69794-050) Establishment Name Address ID/FEI Business Operations Patheon Inc. 205475333 MANUFACTURE(69794-050) , PACK(69794-050) , LABEL(69794-050) Establishment Name Address ID/FEI Business Operations IOI Oleo GmbH 342589974 API MANUFACTURE(69794-050)
Trademark Results [DOJOLVI]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DOJOLVI 88707194 not registered Live/Pending |
Ultragenyx Pharmaceutical Inc. 2019-11-26 |
 DOJOLVI 88352451 not registered Live/Pending |
Ultragenyx Pharmaceutical Inc. 2019-03-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.