Cycloserine by Macleods Pharmaceuticals Limited CYCLOSERINE capsule
Cycloserine by
Drug Labeling and Warnings
Cycloserine by is a Prescription medication manufactured, distributed, or labeled by Macleods Pharmaceuticals Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HEALTH CARE PROVIDER LETTER SECTION
Subject: Temporary importation of cycloSERINE, 250 mg capsules to address shortage
January 07, 2019
Dear Healthcare Professional,
Due to an anticipated shortage of SEROMYCIN® - cycloSERINE capsules, 250mg in the United States (U.S.) market by the end of 2018, Macleods Pharmaceuticals Limited, India (Macleods) is co-ordinating with the U.S. Food and Drug Administration (FDA) to increase the availability of cycloSERINE capsules, 250mg for patients in the United States. Macleods has initiated temporary importation of cycloSERINE capsules, 250 mg into the United States market. This drug product is marketed globally and is manufactured in India.
At this time no other entity except Macleods is authorized by the FDA to import cycloSERINE capsules, 250 mg in the United States. FDA has not approved Macleods cycloSERINE capsules, 250 mg in the United States.
Effective immediately, and during this temporary period, Macleods will offer the following version:
Product Name and Description
Quantity/Packaging
NDC
cycloSERINE Capsules, 250mg
30 Capsules, Non-Child Resistant
Packs of 3x10
NDC: 33342-462-06
It is important to note the following:
The imported product and U.S. FDA-approved product (SEROMYCIN®) contain the same active ingredient (cycloSERINE), strength (250 mg), and dosage form (capsules).
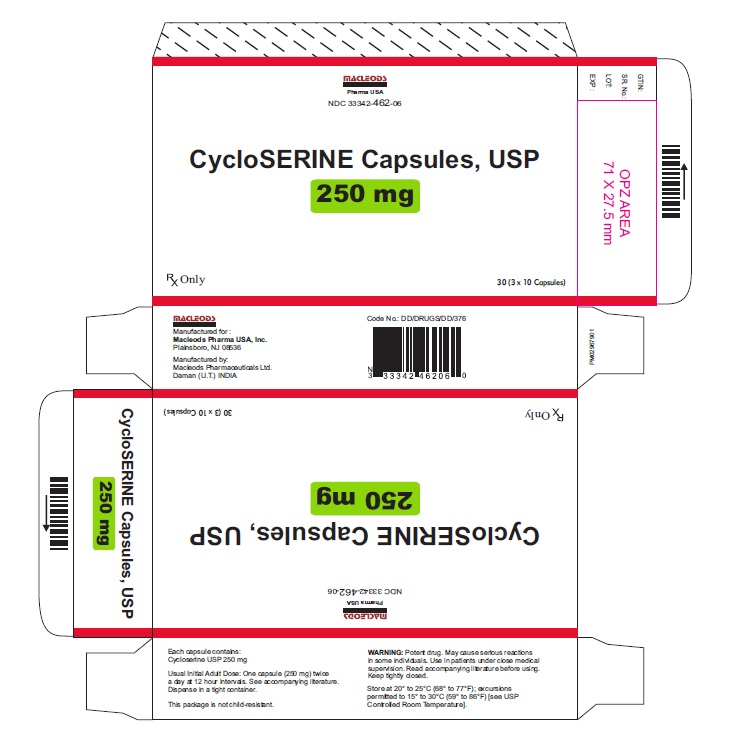
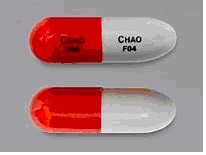
Please note the difference in the imported capsules’ appearance. The imported cycloSERINE capsule is maroon-colored. The U.S.FDA-approved SEROMYCIN® (cycloSERINE) capsule has an opaque red cap and opaque grey body and is imprinted with “CHAO” and “F04”.
U.S. FDA Approved Product
Imported Product
Seromycin (cycloSERINE) capsule, 250 mg
cycloSERINE capsule, 250 mg

The barcode may not register accurately on the U.S. scanning systems. Alternative procedures should be followed to assure that the correct drug product is being used and administered to individual patients. For example, institutions should consider manually inputting the product into their systems and confirm that barcode systems do no provide incorrect information when the product is scanned.
Please refer to the package insert for the FDA-approved cycloSERINE capsules for full prescribing information, including the Warning section for risk of CNS toxicity.
The product comparison table below highlights the differences between the FDA-approved SEROMYCIN (cycloSERINE) capsules and the imported cycloSERINE capsules.
To place an order, or if you have any questions about the information contained in this letter or the use of cycloSERINE capsules, 250 mg, please contact Macleods at 1-888-943-3210 between the hours of 8 a.m. and 5 p.m. (ET), or email at safety@macleodspharma.com.
To report adverse events or quality problems associated with the use of this product, please call Macleods at 1-888-943-3210 between the hours of 8 a.m. and 5 p.m. (ET), or email at safety@macleodspharma.com.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
Complete and submit the report Online: www.fda.gov/medwatch/report.htm
Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htmor call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178 (1-800-332-0178)We urge you to contact our Medical Information Department at 1-888-943-3210 between the hours of 8 a.m. and 5 p.m. (ET), or email safety@macleodspharma.com. If you have any questions about the information contained in this letter or the safe and effective use of CycloSERINE capsules.
Sincerely,
Dr Ashish Mungatiwar
Executive President
Medical Services
Macleods Pharmaceuticals
Comparison Table

- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CYCLOSERINE
cycloserine capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 33342-462 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CYCLOSERINE (UNII: 95IK5KI84Z) (CYCLOSERINE - UNII:95IK5KI84Z) CYCLOSERINE 250 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM OXIDE (UNII: 3A3U0GI71G) TALC (UNII: 7SEV7J4R1U) GELATIN (UNII: 2G86QN327L) SODIUM LAURYL SULFATE (UNII: 368GB5141J) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) CARMOISINE (UNII: DR4641L47F) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Product Characteristics Color RED (Maroon cap) , RED (Maroon body) Score no score Shape CAPSULE Size 19mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 33342-462-06 3 in 1 CARTON 01/18/2019 1 NDC: 33342-462-66 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 01/18/2019 Labeler - Macleods Pharmaceuticals Limited (862128535)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.