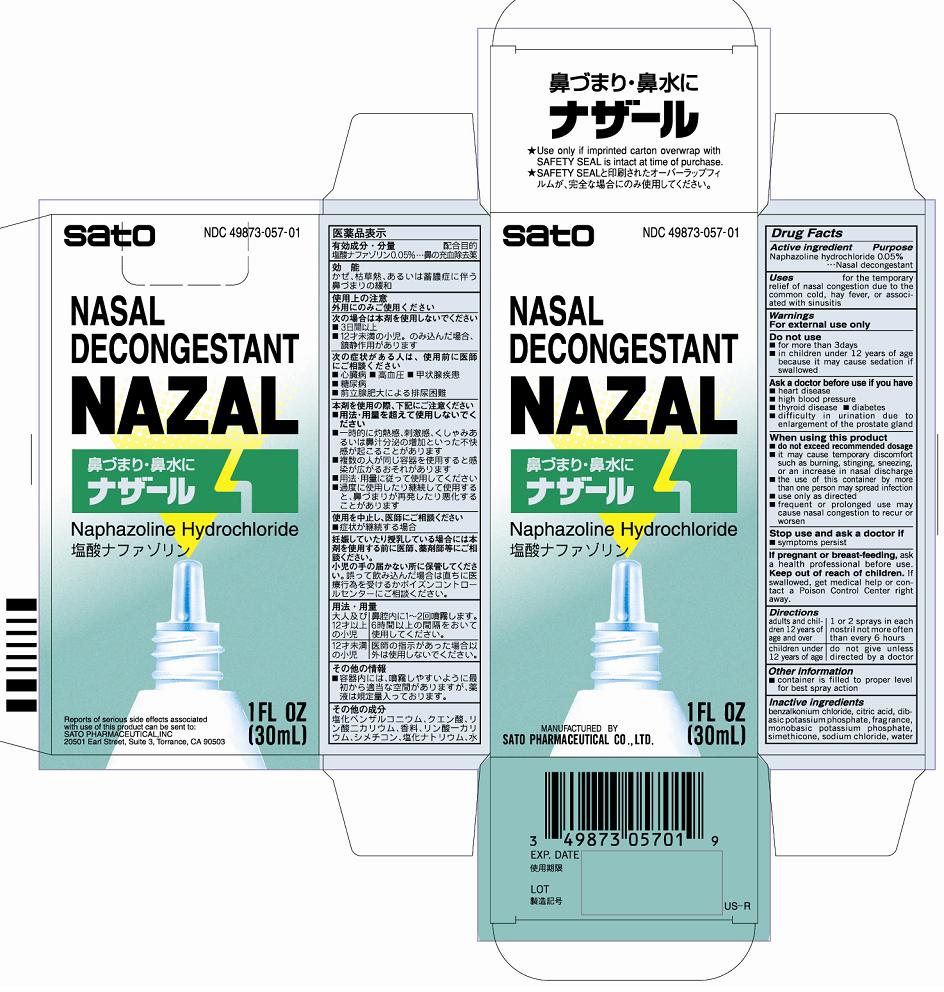
NAZAL- naphazoline hydrochloride liquid
Nazal by
Drug Labeling and Warnings
Nazal by is a Otc medication manufactured, distributed, or labeled by Sato Pharmaceutical Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use onlyDo not use
■for more than 3 days
■in children under 12 years of age because it may cause sedation if swallowedAsk a doctor before use if you have
■heart disease
■high blood pressure
■thyroid disease ■diabetes
■difficulty in urination due to enlargement of the prostate glandWhen using this product
■do not exceed recommended dosage
■if may cause temporary discomfort such as burning, stinging, sneezing, or an increase in nasal discharge
■the use of this container by more than one person may spread infection
■use only as directed
■frequent or prolonged use may cause nasal congestion to recur or worsen - DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NAZAL
naphazoline hydrochloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 49873-057 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Naphazoline hydrochloride (UNII: MZ1131787D) (Naphazoline - UNII:H231GF11BV) Naphazoline hydrochloride 50 mg in 100 mL Inactive Ingredients Ingredient Name Strength benzalkonium chloride (UNII: F5UM2KM3W7) citric acid monohydrate (UNII: 2968PHW8QP) potassium phosphate, dibasic (UNII: CI71S98N1Z) potassium phosphate, monobasic (UNII: 4J9FJ0HL51) dimethicone (UNII: 92RU3N3Y1O) silicon dioxide (UNII: ETJ7Z6XBU4) sodium chloride (UNII: 451W47IQ8X) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49873-057-01 1 in 1 CARTON 06/11/1990 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 06/11/1990 Labeler - Sato Pharmaceutical Co., Ltd. (690575642) Establishment Name Address ID/FEI Business Operations Sato Pharmaceutical Co., Ltd. 715699133 manufacture(49873-057) , label(49873-057) , pack(49873-057)
Trademark Results [Nazal]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NAZAL 73380119 not registered Dead/Abandoned |
SATO PHARMACEUTICAL CO., LTD. 1982-08-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.