GLOSTRIPS- rose bengal sodium strip
GloStrips by
Drug Labeling and Warnings
GloStrips by is a Prescription medication manufactured, distributed, or labeled by Nomax Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- DESCRIPTION
- ACTION
-
INDICATIONS
Rose Bengal ophthalmic strips are used as a diagnostic agent in routine ocular examinations or when superficial conjunctiva or corneal tissue change is suspected. Rose Bengal ophthalmic strips can also serve as an effective aid in the diagnosis of keratoconjunctivitis sicca, keratitis, abrasions or corrosions as well as the detection of foreign bodies.
- CONTRAINDICATIONS
-
DIRECTIONS FOR USE
To ensure optimal staining and patient comfort, the GloStrip impregnated tip should be moistened with one or two drops of sterile, isotonic saline or irrigating solution before application. Shake off excess solution and touch conjunctiva or fornix as required with moistened tip. It is recommended that the patient blink several times after application.
- NOTE
- HOW SUPPLIED
-
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR OPENING STERILE GLOSTRIPS® 1.Grasp free tab ends of wrapping and slowly pull apart. When the white paper handle becomes visible, remove the GloStrip® from the envelope. Fig. 1
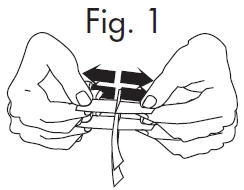
An Alternate Method of Opening: 1.Grasp envelope firmly with two hands as shown in Fig. 2 below. Tear the envelope from both its edges to the strip handle. Fig. 2
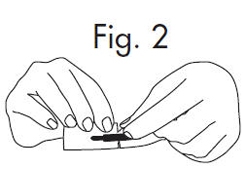
2.Hold the handle end of the GloStrip® with the left hand and the paper envelope without holding the tip with the right hand. See Fig. 3 Fig. 3
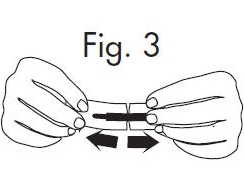
3.Separate the envelope at the tear, exposing the GloStrip® tip. See Fig. 4
Fig. 4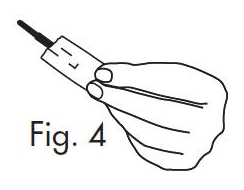
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 100 Strip Carton
NDC: 51801-004-40
Amcon®
Laboratories, Inc.Rose Bengal
GloStrips®
1.3 mg Rose Bengal
Ophthalmic Strips USP
100 Sterile Strips
-
INGREDIENTS AND APPEARANCE
GLOSTRIPS
rose bengal sodium stripProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 51801-004 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Rose Bengal Sodium (UNII: 956575SN5L) (Rose Bengal AT - UNII:1IID5XA5GB) Rose Bengal Sodium 1.3 mg Product Characteristics Color PINK, WHITE Score Shape RECTANGLE (with tapered end) Size 52mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51801-004-40 100 in 1 CARTON 08/20/2013 1 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 08/20/2013 Labeler - Nomax Inc. (103220273) Establishment Name Address ID/FEI Business Operations Nomax Inc. 103220273 MANUFACTURE(51801-004)
Trademark Results [GloStrips]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 GLOSTRIPS 78139112 2760506 Live/Registered |
Amcon Laboratories, Inc. 2002-06-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.