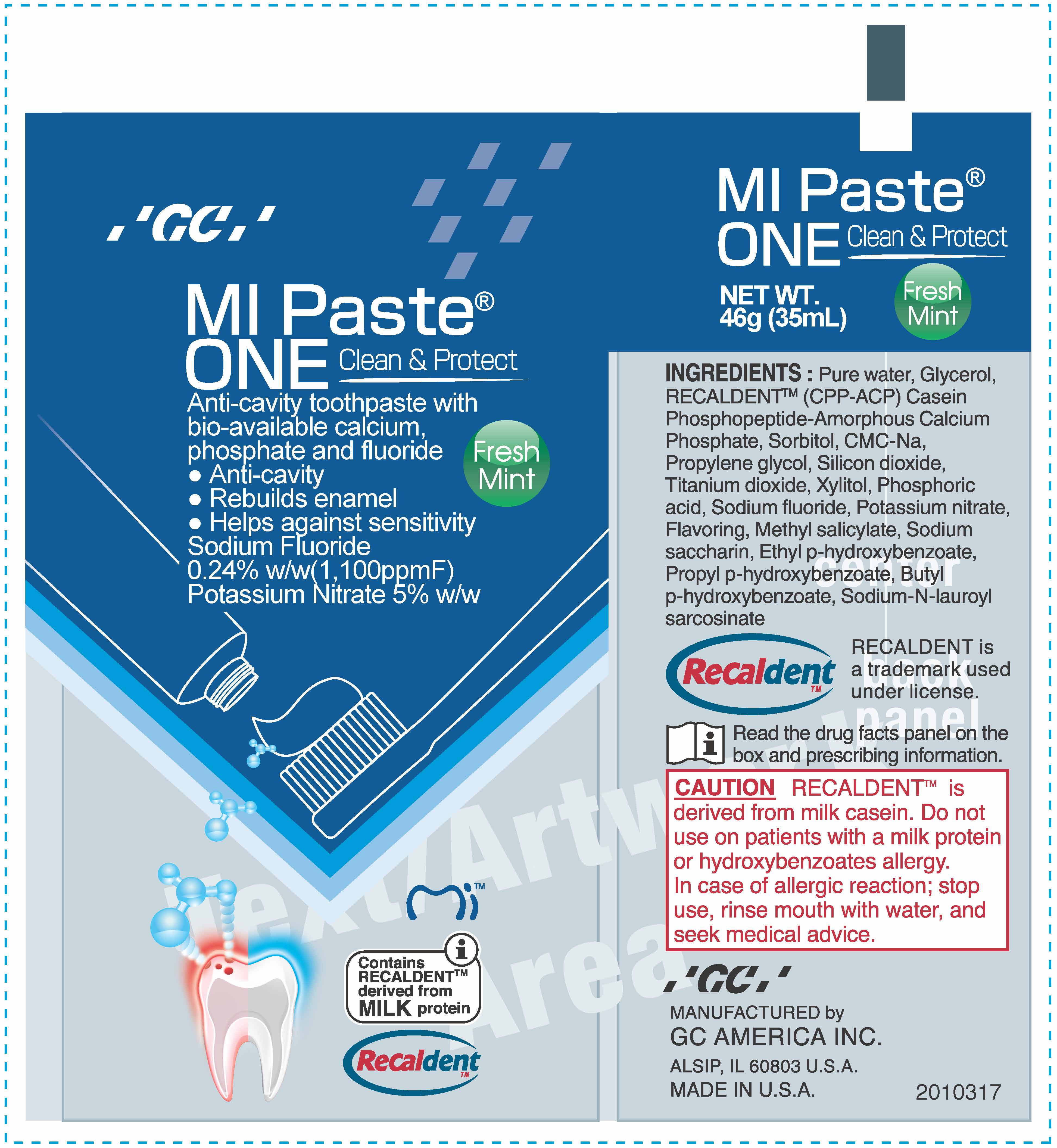
MIPASTE ONE- sodium fluoride, potassium nitrate paste, dentifrice
MIPaste by
Drug Labeling and Warnings
MIPaste by is a Otc medication manufactured, distributed, or labeled by GC America Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredient
- Use
-
Warning
Warning If the sensitivity persists after 4 weeks of use, consult a dentist or physician. Keep out of reach of children. If more than the recommended amount of paste used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away. Do not use on patients with a milk protein and/or hydroxybenzoates allergy. In case of allergic reaction, stop use, rinse mouth with water and seek medical advice.
- Directions
-
Inactive ingredient
Pure water, Glycerol, RECALDENT TM (CPP-ACP) Casein Phosphopeptide-Amorphous Calcium Phosphate, Sorbitol, CMC-Na, Propylene glycol, Silicon dioxide, Titanium dioxide, Xylitol, Phosphoric acid, flavoring, Methyl salicylate, Sodium saccharin, Ethyl p-hydroxybenzoate, Propyl p-hydroxybenzoate, Butyl p-hydroxybenzoate, Sodium-N-lauroyl sarcosinate
- Question or comments
- Warning
- PURPOSE
- Tube box
- Tube
-
INGREDIENTS AND APPEARANCE
MIPASTE ONE
sodium fluoride, potassium nitrate paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 61596-437 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.0024 g in 1 g POTASSIUM NITRATE (UNII: RU45X2JN0Z) (NITRATE ION - UNII:T93E9Y2844) POTASSIUM NITRATE 0.06 g in 1 g Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITOL (UNII: 506T60A25R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) XYLITOL (UNII: VCQ006KQ1E) PHOSPHORIC ACID (UNII: E4GA8884NN) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) ETHYLPARABEN (UNII: 14255EXE39) BUTYLPARABEN (UNII: 3QPI1U3FV8) SACCHARIN SODIUM (UNII: SB8ZUX40TY) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) SODIUM LAUROYL SARCOSINATE (UNII: 632GS99618) METHYL SALICYLATE (UNII: LAV5U5022Y) CALCIUM PHOSPHATE (UNII: 97Z1WI3NDX) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) Product Characteristics Color Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 61596-437-41 1 in 1 BOX 07/03/2017 1 NDC: 61596-437-40 46 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 07/03/2017 Labeler - GC America Inc. (005473608) Establishment Name Address ID/FEI Business Operations GC America Inc. 005473608 manufacture(61596-437)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.