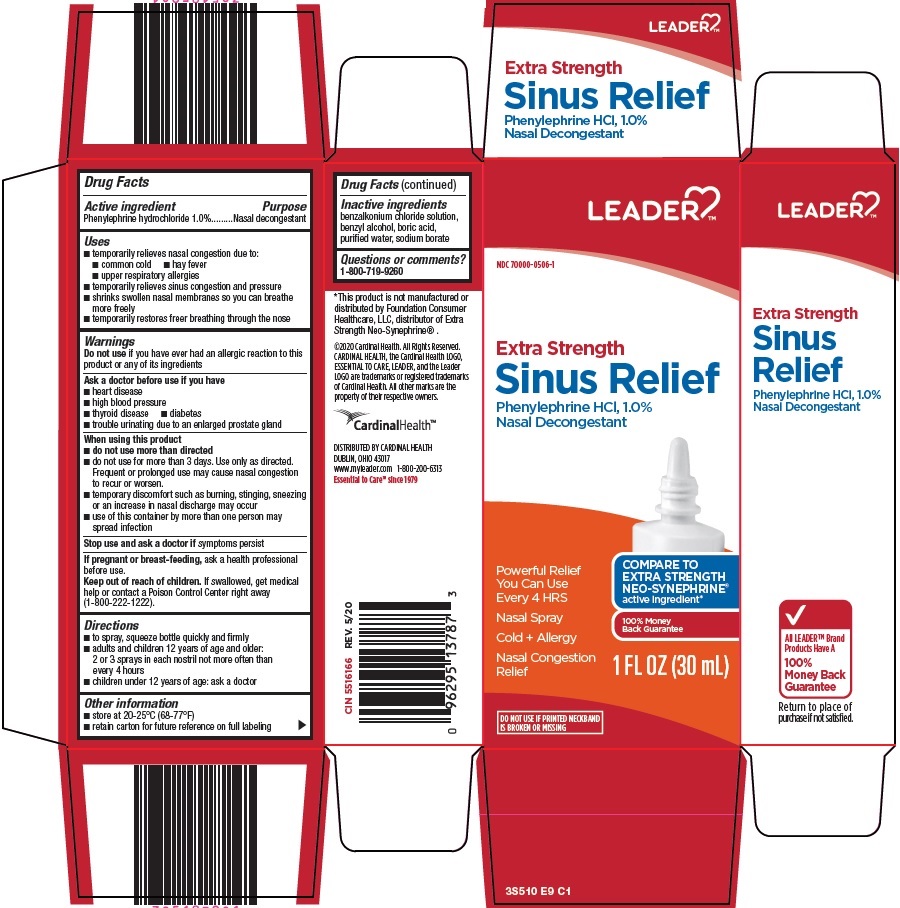
Cardinal Health Sinus Relief Drug Facts
leader sinus relief by
Drug Labeling and Warnings
leader sinus relief by is a Otc medication manufactured, distributed, or labeled by Cardinal Health 110, LLC. dba Leader. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
LEADER SINUS RELIEF- phenylephrine hydrochloride spray
Cardinal Health 110, LLC. dba Leader
----------
Cardinal Health Sinus Relief Drug Facts
Uses
- temporarily relieves nasal congestion due to:
- common cold
- hay fever
- upper respiratory allergies
- temporarily relieves sinus congestion and pressure
- shrinks swollen nasal membranes so you can breathe more freely
- temporarily restores freer breathing through the nose
Warnings
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
When using this product
- do not use more than directed
- do not use for more than 3 days. Use only as directed. Frequent or prolonged use may cause nasal congestion to recur or worsen.
- temporary discomfort such as burning, stinging, sneezing or an increase in nasal discharge may occur
- use of this container by more than one person may spread infection
Directions
- to spray, squeeze bottle quickly and firmly
- adults and children 12 years of age and older: 2 or 3 sprays in each nostril not more often than every 4 hours
- children under 12 years of age: ask a doctor
Inactive ingredients
benzalkonium chloride solution, benzyl alcohol, boric acid, purified water, sodium borate
Package/Label Principal Display Panel
Extra Strength
Sinus Relief
Phenylephrine HCl, 1.0%
Nasal Decongestant
Powerful Relief You Can Use Every 4 HRS
COMPARE TO EXTRA STRENGTH NEO-SYNEPHRINE®
active ingredient
Nasal Spray
Cold + Allergy
100% Money Back Guarantee
Nasal Congestion Relief
1 FL OZ (30 mL)
DO NOT USE IF PRINTED NECKBAND IS BROKEN OR MISSING
| LEADER SINUS RELIEF
phenylephrine hydrochloride spray |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Cardinal Health 110, LLC. dba Leader (063997360) |
Revised: 1/2024
Document Id: dc005d99-11dd-4df8-bf88-101ce97901d3
Set id: 4d63213e-afb6-4f71-a2d8-d1bed6eaa122
Version: 3
Effective Time: 20240111